A microfluidic-based approach to investigate the inflammatory response of macrophages to pristine and drug-loaded nanostructured hydroxyapatite
- PMID: 35865408
- PMCID: PMC9294551
- DOI: 10.1016/j.mtbio.2022.100351
A microfluidic-based approach to investigate the inflammatory response of macrophages to pristine and drug-loaded nanostructured hydroxyapatite
Abstract
The in vitro biological characterization of biomaterials is largely based on static cell cultures. However, for highly reactive biomaterials such as calcium-deficient hydroxyapatite (CDHA), this static environment has limitations. Drastic alterations in the ionic composition of the cell culture medium can negatively affect cell behavior, which can lead to misleading results or data that is difficult to interpret. This challenge could be addressed by a microfluidics-based approach (i.e. on-chip), which offers the opportunity to provide a continuous flow of cell culture medium and a potentially more physiologically relevant microenvironment. The aim of this work was to explore microfluidic technology for its potential to characterize CDHA, particularly in the context of inflammation. Two different CDHA substrates (chemically identical, but varying in microstructure) were integrated on-chip and subsequently evaluated. We demonstrated that the on-chip environment can avoid drastic ionic alterations and increase protein sorption, which was reflected in cell studies with RAW 264.7 macrophages. The cells grown on-chip showed a high cell viability and enhanced proliferation compared to cells maintained under static conditions. Whereas no clear differences in the secretion of tumor necrosis factor alpha (TNF-α) were found, variations in cell morphology suggested a more anti-inflammatory environment on-chip. In the second part of this study, the CDHA substrates were loaded with the drug Trolox. We showed that it is possible to characterize drug release on-chip and moreover demonstrated that Trolox affects the TNF-α secretion and morphology of RAW 264.7 cells. Overall, these results highlight the potential of microfluidics to evaluate (bioactive) biomaterials, both in pristine form and when drug-loaded. This is of particular interest for the latter case, as it allows the biological characterization and assessment of drug release to take place under the same dynamic in vitro environment.
Keywords: Biomaterial; Calcium phosphate cement; Drug release; In vitro; Macrophage; On-chip.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



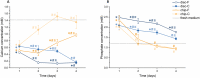

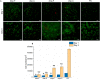


References
-
- Ginebra M.-P., Montufar E.B. second ed. Woodhead Publishing; 2019. Cements as Bone Repair Materials.
-
- Ginebra M.P., Traykova T., Planell J.A. Biomater. 2006;27:2171–2177. - PubMed
-
- Espanol M., Perez R.A., Montufar E.B., Marichal C., Sacco A., Ginebra M.P. Acta Biomater. 2009;5:2752–2762. - PubMed
-
- Ginebra M.-P., Canal C., Espanol M., Pastorino D., Montufar E.B. Adv. Drug Deliv. Rev. 2012;64:1090–1110. - PubMed
-
- Anderson J.M. Annu. Rev. Mater. Res. 2001;31:81–110.
LinkOut - more resources
Full Text Sources

