Growth hormone receptor antagonism downregulates ATP-binding cassette transporters contributing to improved drug efficacy against melanoma and hepatocarcinoma in vivo
- PMID: 35865483
- PMCID: PMC9296106
- DOI: 10.3389/fonc.2022.936145
Growth hormone receptor antagonism downregulates ATP-binding cassette transporters contributing to improved drug efficacy against melanoma and hepatocarcinoma in vivo
Abstract
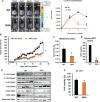
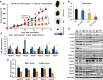
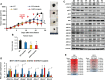
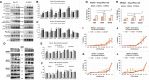
Knockdown of GH receptor (GHR) in melanoma cells in vitro downregulates ATP-binding cassette-containing (ABC) transporters and sensitizes them to anti-cancer drug treatments. Here we aimed to determine whether a GHR antagonist (GHRA) could control cancer growth by sensitizing tumors to therapy through downregulation of ABC transporters in vivo. We intradermally inoculated Fluc-B16-F10 mouse melanoma cells into GHA mice, transgenic for a GHR antagonist (GHRA), and observed a marked reduction in tumor size, mass and tumoral GH signaling. Moreover, constitutive GHRA production in the transgenic mice significantly improved the response to cisplatin treatment by suppressing expression of multiple ABC transporters and sensitizing the tumors to the drug. We confirmed that presence of a GHRA and not a mere absence of GH is essential for this chemo-sensitizing effect using Fluc-B16-F10 allografts in GH knockout (GHKO) mice, where tumor growth was reduced relative to that in GH-sufficient controls but did not sensitize the tumor to cisplatin. We extended our investigation to hepatocellular carcinoma (HCC) using human HCC cells in vitro and a syngeneic mouse model of HCC with Hepa1-6 allografts in GHA mice. Gene expression analyses and drug-efflux assays confirm that blocking GH significantly suppresses the levels of ABC transporters and improves the efficacy of sorafenib towards almost complete tumor clearance. Human patient data for melanoma and HCC show that GHR RNA levels correlate with ABC transporter expression. Collectively, our results validate in vivo that combination of a GHRA with currently available anti-cancer therapies can be effective in attacking cancer drug resistance.
Keywords: ABC transporters; GHA; HCC; drug resistance; growth hormone (GH); growth hormone receptor antagonist (GHRA); melanoma.
Copyright © 2022 Basu, Qian, Mathes, Terry, Arnett, Riddell, Stevens, Funk, Bell, Bokal, Batten, Smith, Mendez-Gibson, Duran-Ortiz, Lach, Mora-Criollo, Kulkarni, Davis, Teaford, Berryman, List, Neggers and Kopchick.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps.Horm Cancer. 2017 Jun;8(3):143-156. doi: 10.1007/s12672-017-0292-7. Epub 2017 Mar 14. Horm Cancer. 2017. PMID: 28293855 Free PMC article.
-
Growth Hormone Upregulates Melanocyte-Inducing Transcription Factor Expression and Activity via JAK2-STAT5 and SRC Signaling in GH Receptor-Positive Human Melanoma.Cancers (Basel). 2019 Sep 12;11(9):1352. doi: 10.3390/cancers11091352. Cancers (Basel). 2019. PMID: 31547367 Free PMC article.
-
Growth Hormone Upregulates Mediators of Melanoma Drug Efflux and Epithelial-to-Mesenchymal Transition In Vitro and In Vivo.Cancers (Basel). 2020 Dec 4;12(12):3640. doi: 10.3390/cancers12123640. Cancers (Basel). 2020. PMID: 33291663 Free PMC article.
-
Role of ATP-binding Cassette Transporters in Sorafenib Therapy for Hepatocellular Carcinoma: An Overview.Curr Drug Targets. 2022;23(1):21-32. doi: 10.2174/1389450122666210412125018. Curr Drug Targets. 2022. PMID: 33845738 Review.
-
Mouse models of growth hormone insensitivity.Rev Endocr Metab Disord. 2021 Mar;22(1):17-29. doi: 10.1007/s11154-020-09600-6. Epub 2020 Oct 10. Rev Endocr Metab Disord. 2021. PMID: 33037595 Free PMC article. Review.
Cited by
-
Exploitation and Verification of a Stroma- and Metastasis-Associated Risk Prognostic Signature in Pancreatic Adenocarcinoma.Pharmaceuticals (Basel). 2022 Oct 28;15(11):1336. doi: 10.3390/ph15111336. Pharmaceuticals (Basel). 2022. PMID: 36355508 Free PMC article.
-
Growth Hormone Upregulates Melanoma Drug Resistance and Migration via Melanoma-Derived Exosomes.Cancers (Basel). 2024 Jul 24;16(15):2636. doi: 10.3390/cancers16152636. Cancers (Basel). 2024. PMID: 39123364 Free PMC article.
-
Growth Hormone Signaling in Bladder Cancer: Transcriptomic Profiling of Patient Samples and In Vitro Evidence of Therapy Resistance via ABC Transporters and EMT Activation.Int J Mol Sci. 2025 Jul 23;26(15):7113. doi: 10.3390/ijms26157113. Int J Mol Sci. 2025. PMID: 40806252 Free PMC article.
-
Nutrition, GH/IGF-1 signaling, and cancer.Endocr Relat Cancer. 2024 Oct 7;31(11):e230048. doi: 10.1530/ERC-23-0048. Print 2024 Nov 1. Endocr Relat Cancer. 2024. PMID: 39166749 Free PMC article. Review.
-
Three E2F target-related genes signature for predicting prognosis, immune features, and drug sensitivity in hepatocellular carcinoma.Front Mol Biosci. 2023 Oct 3;10:1266515. doi: 10.3389/fmolb.2023.1266515. eCollection 2023. Front Mol Biosci. 2023. PMID: 37854038 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

