ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk
- PMID: 35865520
- PMCID: PMC9294453
- DOI: 10.3389/fimmu.2022.873067
ACTIVATE-2: A Double-Blind Randomized Trial of BCG Vaccination Against COVID-19 in Individuals at Risk
Erratum in
-
Corrigendum: ACTIVATE-2: A double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk.Front Immunol. 2022 Aug 31;13:1018384. doi: 10.3389/fimmu.2022.1018384. eCollection 2022. Front Immunol. 2022. PMID: 36119100 Free PMC article.
Abstract
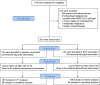
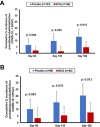
In a recent study of our group with the acronym ACTIVATE, Bacillus Calmete-Guérin (BCG) vaccination reduced the occurrence of new infections compared to placebo vaccination in the elderly. Most benefit was found for respiratory infections. The ACTIVATE-2 study was launched to assess the efficacy of BCG vaccination against coronavirus disease 2019 (COVID-19). In this multicenter, double-blind trial, 301 volunteers aged 50 years or older were randomized (1:1) to be vaccinated with BCG or placebo. The trial end points were the incidence of COVID-19 and the presence of anti-severe acute respiratory syndrome coronavirus 2 (anti-SARS-CoV-2) antibodies, which were both evaluated through 6 months after study intervention. Results revealed 68% relative reduction of the risk to develop COVID-19, using clinical criteria or/and laboratory diagnosis, in the group of BCG vaccine recipients compared with placebo-vaccinated controls, during a 6-month follow-up (OR 0.32, 95% CI 0.13-0.79). In total, eight patients were in need of hospitalization for COVID-19: six in the placebo group and two in the BCG group. Three months after study intervention, positive anti-SARS-CoV-2 antibodies were noted in 1.3% of volunteers in the placebo group and in 4.7% of participants in BCG-vaccinated group. These data indicate that BCG vaccination confers some protection against possible COVID-19 among patients older than 50 years with comorbidities. BCG vaccination may be a promising approach against the COVID-19 pandemic.
Keywords: BCG; COVID-19; SARS-CoV-2; elderly vaccination; trained immunity.
Copyright © 2022 Tsilika, Taks, Dolianitis, Kotsaki, Leventogiannis, Damoulari, Kostoula, Paneta, Adamis, Papanikolaou, Stamatelopoulos, Bolanou, Katsaros, Delavinia, Perdios, Pandi, Tsiakos, Proios, Kalogianni, Delis, Skliros, Akinosoglou, Perdikouli, Poulakou, Milionis, Athanassopoulou, Kalpaki, Efstratiou, Perraki, Papadopoulos, Netea and Giamarellos-Bourboulis.
Conflict of interest statement
EJGB has received honoraria from Abbott CH, InflaRx GmbH, MSD Greece, Sobi Greece and XBiotech Inc.; independent educational grants from AbbVie, Abbott, AxisShield, bioMérieux Inc, InflaRx GmbH, Sobi and XBiotech Inc; and funding from the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grants ImmunoSep and RISKinCOVID (granted to the Hellenic Institute for the Study of Sepsis). MGN was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. MN is a scientific founder of TTxD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous