Mast Cell and Basophil Granule Proteases - In Vivo Targets and Function
- PMID: 35865537
- PMCID: PMC9294451
- DOI: 10.3389/fimmu.2022.918305
Mast Cell and Basophil Granule Proteases - In Vivo Targets and Function
Abstract
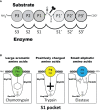
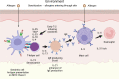
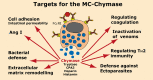
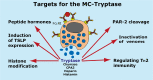
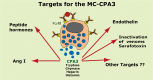
Proteases are stored in very large amounts within abundant cytoplasmic granules of mast cells (MCs), and in lower amounts in basophils. These proteases are stored in their active form in complex with negatively charged proteoglycans, such as heparin and chondroitin sulfate, ready for rapid release upon MC and basophil activation. The absolute majority of these proteases belong to the large family of chymotrypsin related serine proteases. Three such enzymes are found in human MCs, a chymotryptic enzyme, the chymase, a tryptic enzyme, the tryptase and cathepsin G. Cathepsin G has in primates both chymase and tryptase activity. MCs also express a MC specific exopeptidase, carboxypeptidase A3 (CPA3). The targets and thereby the functions of these enzymes have for many years been the major question of the field. However, the fact that some of these enzymes have a relatively broad specificity has made it difficult to obtain reliable information about the biologically most important targets for these enzymes. Under optimal conditions they may cleave a relatively large number of potential targets. Three of these enzymes, the chymase, the tryptase and CPA3, have been shown to inactivate several venoms from snakes, scorpions, bees and Gila monster. The chymase has also been shown to cleave several connective tissue components and thereby to be an important player in connective tissue homeostasis. This enzyme can also generate angiotensin II (Ang II) by cleavage of Ang I and have thereby a role in blood pressure regulation. It also display anticoagulant activity by cleaving fibrinogen and thrombin. A regulatory function on excessive TH2 immunity has also been observed for both the chymase and the tryptase by cleavage of a highly selective set of cytokines and chemokines. The chymase also appear to have a protective role against ectoparasites such as ticks, mosquitos and leeches by the cleavage of their anticoagulant proteins. We here review the data that has accumulated concerning the potential in vivo functions of these enzymes and we discuss how this information sheds new light on the role of MCs and basophils in health and disease.
Keywords: CPA3; chymase; mast cell; serine protease; tryptase.
Copyright © 2022 Hellman, Akula, Fu and Wernersson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
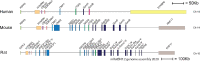



References
-
- Angerth T, Huang RY, Aveskogh M, Pettersson I, Kjellen L, Hellman L. Cloning and Structural Analysis of a Gene Encoding a Mouse Mastocytoma Proteoglycan Core Protein; Analysis of its Evolutionary Relation to Three Cross Hybridizing Regions in the Mouse Genome. Gene (1990) 93(2):235–40. doi: 10.1016/0378-1119(90)90230-O - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

