COVID-19 Vaccination and the Rate of Immune and Autoimmune Adverse Events Following Immunization: Insights From a Narrative Literature Review
- PMID: 35865539
- PMCID: PMC9294236
- DOI: 10.3389/fimmu.2022.872683
COVID-19 Vaccination and the Rate of Immune and Autoimmune Adverse Events Following Immunization: Insights From a Narrative Literature Review
Abstract
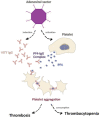
Despite their proven efficacy and huge contribution to the health of humankind, vaccines continue to be a source of concern for some individuals around the world. Vaccinations against COVID-19 increased the number of distressed people and intensified their distrust, particularly as the pandemic was still emerging and the populations were encouraged to be vaccinated under various slogans like "back to normal life" and "stop coronavirus", goals which are still to be achieved. As fear of vaccination-related adverse events following immunization (AEFIs) is the main reason for vaccine hesitancy, we reviewed immune and autoimmune AEFIs in particular, though very rare, as the most worrisome aspect of the vaccines. Among others, autoimmune AEFIs of the most commonly administered COVID-19 vaccines include neurological ones such as Guillain-Barre syndrome, transverse myelitis, and Bell's palsy, as well as myocarditis. In addition, the newly introduced notion related to COVID-19 vaccines, "vaccine-induced immune thrombotic thrombocytopenia/vaccine-induced prothrombotic immune thrombotic thrombocytopenia" (VITT/VIPITT)", is of importance as well. Overviewing recent medical literature while focusing on the major immune and autoimmune AEFIs, demonstrating their rate of occurrence, presenting the cases reported, and their link to the specific type of COVID-19 vaccines represented the main aim of our work. In this narrative review, we illustrate the different vaccine types in current use, their associated immune and autoimmune AEFIs, with a focus on the 3 main COVID-19 vaccines (BNT162b2, mRNA-1273, and ChAdOx1). While the rate of AEFIs is extremely low, addressing the issue in this manner, in our opinion, is the best strategy for coping with vaccine hesitancy.
Keywords: COVID-19 vaccine; Guillain-Barre syndrome; autoimmune side effects; myocarditis; vaccine hesitancy; vaccine-induced immune thrombotic thrombocytopenia.
Copyright © 2022 Mahroum, Lavine, Ohayon, Seida, Alwani, Alrais, Zoubi and Bragazzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Serious neurological adverse events following immunization against SARS-CoV-2: a narrative review of the literature.Ther Adv Drug Saf. 2023 May 21;14:20420986231165674. doi: 10.1177/20420986231165674. eCollection 2023. Ther Adv Drug Saf. 2023. PMID: 37223456 Free PMC article. Review.
-
Safety of COVID-19 vaccination and acute neurological events: A self-controlled case series in England using the OpenSAFELY platform.Vaccine. 2022 Jul 30;40(32):4479-4487. doi: 10.1016/j.vaccine.2022.06.010. Epub 2022 Jun 7. Vaccine. 2022. PMID: 35715350 Free PMC article.
-
Disproportionality analysis of adverse neurological and psychiatric reactions with the ChAdOx1 (Oxford-AstraZeneca) and BNT162b2 (Pfizer-BioNTech) COVID-19 vaccines in the United Kingdom.Expert Opin Drug Saf. 2023 Apr;22(4):343-349. doi: 10.1080/14740338.2022.2120607. Epub 2022 Sep 7. Expert Opin Drug Saf. 2023. PMID: 36043937
-
Active Safety Surveillance of Four Types of COVID-19 Vaccines: A National Study from Jordan.Clin Drug Investig. 2022 Oct;42(10):813-827. doi: 10.1007/s40261-022-01191-1. Epub 2022 Aug 23. Clin Drug Investig. 2022. PMID: 35999428 Free PMC article.
-
The Ambivalence of Post COVID-19 Vaccination Responses in Humans.Biomolecules. 2024 Oct 17;14(10):1320. doi: 10.3390/biom14101320. Biomolecules. 2024. PMID: 39456253 Free PMC article. Review.
Cited by
-
ASIA Syndrome: State-of-the-Art and Future Perspectives.Vaccines (Basel). 2024 Oct 17;12(10):1183. doi: 10.3390/vaccines12101183. Vaccines (Basel). 2024. PMID: 39460349 Free PMC article. Review.
-
Cutaneous Adverse Reactions to SARS-CoV-2 Vaccines: A Systematic Review and Meta-Analysis.Vaccines (Basel). 2022 Sep 6;10(9):1475. doi: 10.3390/vaccines10091475. Vaccines (Basel). 2022. PMID: 36146553 Free PMC article. Review.
-
SARS-CoV-2 and monkeypox: what is common and what is not in a present pandemic versus a potential one-a neuropsychiatric narrative review.Egypt J Neurol Psychiatr Neurosurg. 2022;58(1):127. doi: 10.1186/s41983-022-00563-w. Epub 2022 Nov 8. Egypt J Neurol Psychiatr Neurosurg. 2022. PMID: 36408294 Free PMC article. Review.
-
Nuclear Factor-κB is a Prime Candidate for the Diagnosis and Control of Inflammatory Cardiovascular Disease.Eur Cardiol. 2023 Jun 7;18:e40. doi: 10.15420/ecr.2023.10. eCollection 2023. Eur Cardiol. 2023. PMID: 37456770 Free PMC article. Review.
-
A case of IgG4-related ophthalmic disease after SARS-CoV-2 vaccination: case report and literature review.Front Immunol. 2024 Feb 22;15:1303589. doi: 10.3389/fimmu.2024.1303589. eCollection 2024. Front Immunol. 2024. PMID: 38455056 Free PMC article. Review.
References
-
- Organization WH . WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 (2020). Available at: https://www.who.int/director-general/speeches/detail/who-director-genera....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

