Targeting Myeloid Checkpoint Molecules in Combination With Antibody Therapy: A Novel Anti-Cancer Strategy With IgA Antibodies?
- PMID: 35865547
- PMCID: PMC9295600
- DOI: 10.3389/fimmu.2022.932155
Targeting Myeloid Checkpoint Molecules in Combination With Antibody Therapy: A Novel Anti-Cancer Strategy With IgA Antibodies?
Erratum in
-
Corrigendum: Targeting myeloid checkpoint molecules in combination with antibody therapy: A novel anti-cancer strategy with IgA antibodies?Front Immunol. 2022 Sep 13;13:1017924. doi: 10.3389/fimmu.2022.1017924. eCollection 2022. Front Immunol. 2022. PMID: 36177025 Free PMC article.
Abstract
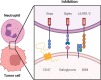
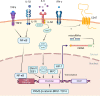
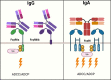
Immunotherapy with therapeutic antibodies has shown a lack of durable responses in some patients due to resistance mechanisms. Checkpoint molecules expressed by tumor cells have a deleterious impact on clinical responses to therapeutic antibodies. Myeloid checkpoints, which negatively regulate macrophage and neutrophil anti-tumor responses, are a novel type of checkpoint molecule. Myeloid checkpoint inhibition is currently being studied in combination with IgG-based immunotherapy. In contrast, the combination with IgA-based treatment has received minimal attention. IgA antibodies have been demonstrated to more effectively attract and activate neutrophils than their IgG counterparts. Therefore, myeloid checkpoint inhibition could be an interesting addition to IgA treatment and has the potential to significantly enhance IgA therapy.
Keywords: CD47-SIRPalpha axis; IgA; antibodies; cancer immonotherapy; immune checkpoint; macrophages; myeloid checkpoints; neutrophils (PMNs).
Copyright © 2022 Chan, Lustig, Baumann, Valerius, van Tetering and Leusen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

