Synthesis, column packing and liquid chromatography of molecularly imprinted polymers for the acid black 1, acid black 210, and acid Brown 703 dyes
- PMID: 35865557
- PMCID: PMC9258683
- DOI: 10.1039/d2ra02357a
Synthesis, column packing and liquid chromatography of molecularly imprinted polymers for the acid black 1, acid black 210, and acid Brown 703 dyes
Abstract
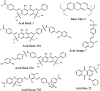
Molecularly imprinted polymers have been synthesized for the acid black 1, acid black 210, and acid brown 703 dyes using methacrylic acid, ethylene glycol, and azobisisobutyronitrile as the monomer, cross-linker, and initiator, respectively, in the ratio of 1 : 10 : 44 (template:monomer:cross-linker). The MIPs were used for the selective removal of their corresponding dyes. The selective nature of the MIPs towards their respective dyes was confirmed by a homemade liquid chromatography system. The resultant polymer materials were packed in a stainless steel column and checked for the separation of mixtures of dyes in liquid chromatography. The dyes complementary in structure to the imprinted cavities in the MIPs had long retention times, showing the highly selective nature of the MIPs. The pH, quantity of the MIPs, time, and concentration of the dyes were optimized for the highly efficient removal of the newly synthesized MIP adsorbents in batch adsorption studies. First-order, second-order, and intra-particle diffusion models were applied to all the three MIP-based adsorbents for their kinetic investigations towards the dyes. All the three MIPs selectively absorbed their target template molecule in the presence of four other template dyes having closely related structures with % RSD < 4% for the three batch experiments. The synthesized MIPs were characterized by FTIR, SEM imaging and liquid chromatography. FTIR results strongly confirmed the presence of hydrogen bonding interactions (600-900) between the template and the individual monomers present in the unwashed MIPs. Liquid chromatography revealed the highly selective nature of the MIPs towards their template molecules. The synthesized polymeric substances possess excellent thermal, chemical, and mechanical stability and can be reused several hundred times. The MIPs were applied in the removal of dyes from spiked water samples (river water, tap water and distilled water) where the % removal of the dyes by their corresponding MIPs was greater than 90%.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Development of a molecularly imprinted polymer for prometryne clean-up in the environment.J Sep Sci. 2013 Dec;36(24):3911-7. doi: 10.1002/jssc.201300914. Epub 2013 Nov 18. J Sep Sci. 2013. PMID: 24151183
-
Chromatographic performance of zidovudine imprinted polymers coated silica stationary phases.Talanta. 2022 Mar 1;239:123115. doi: 10.1016/j.talanta.2021.123115. Epub 2021 Nov 30. Talanta. 2022. PMID: 34890940
-
Synthesis and evaluation of molecularly imprinted polymers with binary functional monomers for the selective removal of perfluorooctanesulfonic acid and perfluorooctanoic acid.J Chromatogr A. 2017 Sep 22;1516:42-53. doi: 10.1016/j.chroma.2017.08.023. Epub 2017 Aug 12. J Chromatogr A. 2017. PMID: 28823786
-
[Research progress of molecularly imprinted polymers in separation of chiral drugs by capillary electrochromatography].Se Pu. 2020 Sep 8;38(9):1046-1056. doi: 10.3724/SP.J.1123.2020.03018. Se Pu. 2020. PMID: 34213271 Review. Chinese.
-
Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials.Molecules. 2021 Sep 16;26(18):5612. doi: 10.3390/molecules26185612. Molecules. 2021. PMID: 34577083 Free PMC article. Review.
Cited by
-
Synthesis and Characterization of MIPs for Selective Removal of Textile Dye Acid Black-234 from Wastewater Sample.Molecules. 2023 Feb 6;28(4):1555. doi: 10.3390/molecules28041555. Molecules. 2023. PMID: 36838543 Free PMC article.
-
Porous Polymers Based on 9,10-Bis(methacryloyloxymethyl)anthracene-Towards Synthesis and Characterization.Materials (Basel). 2023 Mar 25;16(7):2610. doi: 10.3390/ma16072610. Materials (Basel). 2023. PMID: 37048904 Free PMC article.
-
Optimization of photodegradation of acid blue 1 dye on aluminosilicate supported Cu doped TiO2 magnetic nanocatalyst using response surface methodology.Sci Rep. 2025 Feb 14;15(1):5550. doi: 10.1038/s41598-025-89968-0. Sci Rep. 2025. PMID: 39952986 Free PMC article.
-
Synthesis, evaluation of drug delivery potential, and the quantum chemical investigation on a molecular imprinted polymer for quetiapine antipsychotic; a joint experimental and density functional theory study.Front Chem. 2022 Oct 14;10:1001685. doi: 10.3389/fchem.2022.1001685. eCollection 2022. Front Chem. 2022. PMID: 36311434 Free PMC article.
References
-
- Kareem O. A. Res. J. Text. Appar. 2012;16:79–92.
-
- Frey M. S. J. Chem. Educ. 1981;58:301–305.
-
- Sharma V. McKone H. T. Markow P. G. J. Chem. Educ. 2011;88:24–28.
-
- Bafana A. Devi S. S. Chakrabarti T. Environ. Rev. 2011;19:350–370.
-
- Gupta V. K. Suhas J. Environ. Manage. 2009;90:2313–2342. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

