Fullerene C70/porphyrin hybrid nanoarchitectures: single-cocrystal nanoribbons with ambipolar charge transport properties
- PMID: 35865602
- PMCID: PMC9258400
- DOI: 10.1039/d2ra02669d
Fullerene C70/porphyrin hybrid nanoarchitectures: single-cocrystal nanoribbons with ambipolar charge transport properties
Abstract
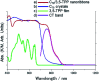
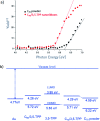
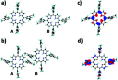
In recent years, supramolecular cocrystals containing organic donors and acceptors have been explored as active components in organic field-effect transistors (FETs). Herein, we report the synthesis of novel single-cocrystal nanoribbons with ambipolar charge transport characteristics from C70 and 5,10,15,20-tetrakis(3,5-dimethoxyphenyl)porphyrin (3,5-TPP) in a 3 : 2 ratio. The C70/3,5-TPP nanoribbons exhibited a new strong absorption band in the near-infrared region, indicating the presence of charge-transfer interactions between C70 and 3,5-TPP in the cocrystals. We elucidated the mechanism of the charge-transport properties of the nanoribbons using photoemission yield spectroscopy in air and theoretical calculations. A strong interaction between porphyrins in the one-dimensional porphyrin chains formed in C70/3,5-TPP nanoribbons, which was confirmed by single-crystal X-ray diffraction, plays a crucial role in their hole transport properties.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Kim F. S. Ren G. Jenekhe S. A. Chem. Mater. 2011;23:682–732. doi: 10.1021/cm102772x. - DOI
LinkOut - more resources
Full Text Sources

