Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey
- PMID: 35865654
- PMCID: PMC9283112
- DOI: 10.1016/j.lana.2022.100235
Achieving high immunogenicity against poliovirus with fractional doses of inactivated poliovirus vaccine in Ecuador-results from a cross-sectional serological survey
Abstract
Background: In January 2018, Ecuador changed its routine immunization schedule by replacing one full dose of inactivated poliovirus vaccine (IPV) administered intramuscularly at 2 months of age with two doses of fractional IPV (1/5th of full dose, fIPV) administered intradermally at 2 and 4 months of age; and bivalent oral poliovirus vaccine (serotypes 1 and 3, bOPV) continues to be used. We compared seroprevalence and titres of polio antibodies achieved by the past and the current immunization schedules.
Methods: This was a cross-sectional serological survey in children in Ecuador who received bOPV and either one IPV dose in 2017 or two fIPV doses in 2018. One blood sample was collected between October 2020 and March 2021 and analysed for presence of poliovirus neutralizing antibodies at CDC, Atlanta by microneutralization assay.
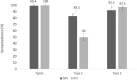
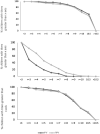
Findings: We obtained 321 analysable samples from 329 (97·6%) enrolled children (160 received IPV and 161 fIPV). For serotype 2, seroprevalence was 50·0% (CI95%= 44·2-55·8%) for IPV and 83·2% (CI95%=78·5-87·1%) for fIPV recipients (p<0·001). Median antibody titers for serotype 2 were significantly lower for IPV than for fIPV recipients (3·0, CI95%= 3 - 3·5 vs. 4·8, CI95%= 4·5 - 5·2, p<0·001). Seroprevalence for serotypes 1 and 3 was above 90% and was not significantly different between IPV and fIPV recipients.
Interpretation: Ecuador achieved significantly better poliovirus serotype 2 immunogenicity with two fIPV doses than with one IPV dose, while preserving vaccine supply and reducing costs. Our data provide further evidence that fIPV is a beneficial and potentially a cost-effective option in polio immunization.
Funding: WHO obtained funds for the study from Rotary International.
Keywords: Ecuador; Fractional dose inactivated poliovirus vaccine; Poliomyelitis; Routine immunization; Seroprevalence assay.
© 2022 World Health Organization.
Conflict of interest statement
None declared, all authors.
Figures
Similar articles
-
Immunogenicity of full and fractional dose of inactivated poliovirus vaccine for use in routine immunisation and outbreak response: an open-label, randomised controlled trial.Lancet. 2019 Jun 29;393(10191):2624-2634. doi: 10.1016/S0140-6736(19)30503-3. Epub 2019 May 16. Lancet. 2019. PMID: 31104832 Free PMC article. Clinical Trial.
-
Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule.Vaccine. 2017 May 19;35(22):2993-2998. doi: 10.1016/j.vaccine.2017.03.008. Epub 2017 Apr 20. Vaccine. 2017. PMID: 28434691 Free PMC article. Review.
-
Evaluating the cost per child vaccinated with full versus fractional-dose inactivated poliovirus vaccine.Vaccine X. 2019 Jul 15;2:100032. doi: 10.1016/j.jvacx.2019.100032. eCollection 2019 Aug 9. Vaccine X. 2019. PMID: 31384747 Free PMC article.
-
Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study.Lancet Infect Dis. 2015 Nov;15(11):1273-82. doi: 10.1016/S1473-3099(15)00219-4. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318714 Clinical Trial.
-
Equivalent schedules of intradermal fractional dose versus intramuscular full dose of inactivated polio vaccine for prevention of poliomyelitis.Cochrane Database Syst Rev. 2019 Dec 19;12(12):CD011780. doi: 10.1002/14651858.CD011780.pub2. Cochrane Database Syst Rev. 2019. PMID: 31858595 Free PMC article.
Cited by
-
Impact of novel Oral Poliovirus Type 2 vaccination campaigns: A seroprevalence survey in Nigeria, 2022.Vaccine. 2025 Apr 30;54:126978. doi: 10.1016/j.vaccine.2025.126978. Epub 2025 Mar 19. Vaccine. 2025. PMID: 40112661 Free PMC article. No abstract available.
-
Evaluating the Impact of Needle-Free Delivery of Inactivated Polio Vaccine on Nigeria's Routine Immunization Program: An Implementation Hybrid Trial.Vaccines (Basel). 2025 May 16;13(5):533. doi: 10.3390/vaccines13050533. Vaccines (Basel). 2025. PMID: 40432142 Free PMC article.
-
The Last Mile in Polio Eradication: Program Challenges and Perseverance.Pathogens. 2024 Apr 15;13(4):323. doi: 10.3390/pathogens13040323. Pathogens. 2024. PMID: 38668278 Free PMC article. Review.
-
Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance.Pathogens. 2024 Mar 4;13(3):224. doi: 10.3390/pathogens13030224. Pathogens. 2024. PMID: 38535567 Free PMC article. Review.
References
-
- Robbins F.C., de Quadros C.A. Certification of the eradication of indigenous transmission of wild poliovirus in the Americas. J Infect Dis. 1997;175(1):S281–S285. - PubMed
-
- Cases of Wild Poliovirus by country and year: Online Reference Table. Retrieved 10/02/2022, from http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpoli....
-
- Minor P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine. 2009;27(20):2649–2652. - PubMed
-
- Kew O.M., Sutter R.W., de Gourville E.M., Dowdle W.R., Pallansch M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Ann Rev Microbiol. 2005;59:587–635. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

