Efficacy of a 3% Kānuka oil cream for the treatment of moderate-to-severe eczema: A single blind randomised vehicle-controlled trial
- PMID: 35865740
- PMCID: PMC9294249
- DOI: 10.1016/j.eclinm.2022.101561
Efficacy of a 3% Kānuka oil cream for the treatment of moderate-to-severe eczema: A single blind randomised vehicle-controlled trial
Abstract
Background: Māori, the indigenous people of New Zealand, have traditionally used the kānuka tree as part of their healing system, Rongoā Māori, and the oil from the kānuka tree has demonstratable anti-inflammatory and anti-bacterial properties. This trial investigated the efficacy and safety of a 3% kānuka oil (KO) cream compared to vehicle control (VC) for the topical treatment of eczema. The trial was conducted through a nationwide community pharmacy research network.
Methods: This single-blind, parallel-group, randomised, vehicle-controlled trial was undertaken in 11 research trained community pharmacies across New Zealand. Eighty adult participants with self-reported moderate-to-severe eczema, assessed by Patient Orientated Eczema Measure (POEM) were randomised by blinded investigators to apply 3% KO cream or VC topically, twice daily, for six weeks. Randomisation was stratified by site and eczema severity, moderate versus severe. Primary outcome was difference in POEM scores at week six between groups by intention to treat. The study is registered on the Australian New Zealand Clinical Trial Registry (ANZCTR) reference number, ACTRN12618001754235.
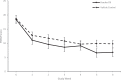
Findings: Eighty participants were recruited between 17 May 2019 and 10 May 2021 (41 KO group, 39 VC group). Mean POEM score (standard deviation) improved between baseline and week six for KO group, 18·4 (4·4) to 6·8 (5·5), and VC group, 18·7 (4·5) to 9·8 (6·5); mean difference between groups (95% confidence interval) was -3·1 (-6·0 to -0·2), p = 0·036. There were three adverse events reported in the KO group related to the intervention and two in the control group.
Interpretation: The KO group had a significant improvement in POEM score compared to VC. Rates of adverse events and withdrawals were similar between groups with no serious adverse events reported. Treatment acceptability was high for both groups across all domains. Our results suggest that in adults with moderate-to-severe eczema, the addition of KO to a daily emollient regimen led to a reduction in POEM score compared to VC. KO may represent an effective, safe, and well tolerated treatment for moderate-to-severe eczema in adults.
Funding: Hikurangi Bioactives (Ruatoria, New Zealand) and HoneyLab (Tauranga, New Zealand), supported by a grant from Callaghan Innovation.
Keywords: Decentralised; Dermatitis; Dermatology; Eczema; Kānuka.
© 2022 The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. R.B., A.S., and N.S. declare funding from Hikuangi Bioactives and Honeylab to the MRINZ for the submitted work. All other authors declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Schmitt J, Langan S, Stamm T, Williams HC, on behalf of the Harmonizing Outcome Measurements in Eczema (HOME) Delphi panel Core outcome domains for controlled trials and clinical recordkeeping in eczema: International multiperspective delphi consensus process. J Invest Dermatol. 2011;131(3):623–630. - PubMed
-
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M. The harmonising outcome measures for eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clinic Immunol. 2014;134(4):800–807. - PubMed
-
- Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

