Reduced Insulin Signaling Targeted to Serotonergic Neurons but Not Other Neuronal Subtypes Extends Lifespan in Drosophila melanogaster
- PMID: 35865744
- PMCID: PMC9294736
- DOI: 10.3389/fnagi.2022.893444
Reduced Insulin Signaling Targeted to Serotonergic Neurons but Not Other Neuronal Subtypes Extends Lifespan in Drosophila melanogaster
Erratum in
-
Corrigendum: Reduced insulin signaling targeted to serotonergic neurons but not other neuronal subtypes extends lifespan in Drosophila melanogaster.Front Aging Neurosci. 2023 Oct 17;15:1307495. doi: 10.3389/fnagi.2023.1307495. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37915830 Free PMC article.
Abstract
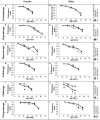
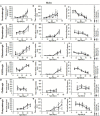
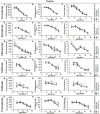
Reduced Insulin/IGF-like signaling (IIS) plays an evolutionarily conserved role in improving longevity and some measures of health-span in model organisms. Recent studies, however, have found a disconnection between lifespan extension and behavioral health-span. We have previously shown that reduction of IIS in Drosophila neurons extends female lifespan but does not improve negative geotaxis senescence and has a detrimental effect on exploratory walking senescence in both sexes. We hypothesize that individual neuronal subtypes respond differently to IIS changes, thus the behavioral outcomes of pan-neuronal IIS reduction are the balance of positive, negative and neutral functional effects. In order to further understand how reduced IIS in neurons independently modulates lifespan and locomotor behavioral senescence we expressed a dominant negative Insulin receptor transgene selectively in individual neuronal subtypes and measured the effects on lifespan and two measures of locomotor senescence, negative geotaxis and exploratory walking. IIS reduction in cholinergic, GABAergic, dopaminergic, glutamatergic, and octopaminergic neurons was found to have either no affect or a detrimental effect on lifespan and locomotor senescence. However, reduction of IIS selectively in serotonergic neurons resulted in extension of lifespan in females with no effect on locomotor senescence. These data indicate that individual neuronal subtypes respond differently to IIS changes in the modulation of lifespan and locomotor senescence, and identify a specific role for the insulin receptor in serotonergic neurons in the modulation of lifespan.
Keywords: Drosophila; ageing; behavioral senescence; insulin/IGF-like signaling; serotonergic neurons.
Copyright © 2022 Dravecz, Shaw, Davies, Brown, Ormerod, Vu, Walker, Taank, Shirras and Broughton.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Since 17th January 2022, the co-author GV has been employed by Frontiers Media SA. GV declared his/her affiliation with Frontiers, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Figures



References
-
- Bhandari P., Jones M. A., Martin I., Grotewiel M. S. (2007). Dietary restriction alters demographic but not behavioral aging in Drosophila. Aging Cell 6 631–637. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

