Miltefosine and Benznidazole Combination Improve Anti- Trypanosoma cruzi In Vitro and In Vivo Efficacy
- PMID: 35865815
- PMCID: PMC9294734
- DOI: 10.3389/fcimb.2022.855119
Miltefosine and Benznidazole Combination Improve Anti- Trypanosoma cruzi In Vitro and In Vivo Efficacy
Abstract
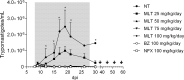
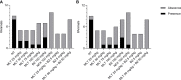
Drug repurposing and combination therapy have been proposed as cost-effective strategies to improve Chagas disease treatment. Miltefosine (MLT), a synthetic alkylphospholipid initially developed for breast cancer and repositioned for leishmaniasis, is a promising candidate against Trypanosoma cruzi infection. This study evaluates the efficacy of MLT as a monodrug and combined with benznidazole (BZ) in both in vitro and in vivo models of infection with T. cruzi (VD strain, DTU TcVI). MLT exhibited in vitro activity on amastigotes and trypomastigotes with values of IC50 = 0.51 µM (0.48 µM; 0,55 µM) and LC50 = 31.17 µM (29.56 µM; 32.87 µM), respectively. Drug interaction was studied with the fixed-ration method. The sum of the fractional inhibitory concentrations (ΣFICs) resulted in ∑FIC= 0.45 for trypomastigotes and ∑FIC= 0.71 for amastigotes, suggesting in vitro synergistic and additive effects, respectively. No cytotoxic effects on host cells were observed. MLT efficacy was also evaluated in a murine model of acute infection alone or combined with BZ. Treatment was well tolerated with few adverse effects, and all treated animals displayed significantly lower mean peak parasitemia and mortality than infected non-treated controls (p<0.05). The in vivo studies showed that MLT led to a dose-dependent parasitostatic effect as monotherapy which could be improved by combining with BZ, preventing parasitemia rebound after a stringent immunosuppression protocol. These results support MLT activity in clinically relevant stages from T. cruzi, and it is the first report of positive interaction with BZ, providing further support for evaluating combined schemes using MLT and exploring synthetic alkylphospholipids as drug candidates.
Keywords: Chagas disease; Trypanosoma cruzi; benznidazole; drug combination chemotherapy; drug repositioning; miltefosine.
Copyright © 2022 Gulin, Bisio, Rocco, Altcheh, Solana and García-Bournissen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

