Between imide, imidyl and nitrene - an imido iron complex in two oxidation states
- PMID: 35865905
- PMCID: PMC9258327
- DOI: 10.1039/d2sc01088g
Between imide, imidyl and nitrene - an imido iron complex in two oxidation states
Abstract
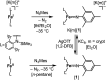
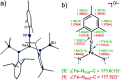
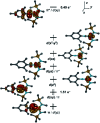
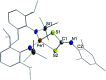
Imidyl and nitrene metal species play an important role in the N-functionalisation of unreactive C-H bonds as well as the aziridination of olefines. We report on the synthesis of the trigonal imido iron complexes [Fe(NMes)L2]0,- (L = -N{Dipp}SiMe3); Dipp = 2,6-diisopropyl-phenyl; Mes = (2,4,6-trimethylphenyl) via reaction of mesityl azide (MesN3) with the linear iron precursors [FeL2]0,-. UV-vis-, EPR-, 57Fe Mössbauer spectroscopy, magnetometry, and computational methods suggest for the reduced form an electronic structure as a ferromagnetically coupled iron(ii) imidyl radical, whereas oxidation leads to mixed iron(iii) imidyl and electrophilic iron(ii) nitrene character. Reactivity studies show that both complexes are capable of H atom abstraction from C-H bonds. Further, the reduced form [Fe(NMes)L2]- reacts nucleophilically with CS2 by inserting into the imido iron bond, as well as electrophilically with CO under nitrene transfer. The neutral [Fe(NMes)L2] complex shows enhanced electrophilic behavior as evidenced by nitrene transfer to a phosphine, yet in combination with an overall reduced reactivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
High-Spin Imido Cobalt Complexes with Imidyl Radical Character*.Angew Chem Int Ed Engl. 2021 Jul 5;60(28):15376-15380. doi: 10.1002/anie.202103841. Epub 2021 Jun 9. Angew Chem Int Ed Engl. 2021. PMID: 33977634 Free PMC article.
-
Umpolung in a Pair of Cobalt(III) Terminal Imido/Imidyl Complexes.Angew Chem Int Ed Engl. 2022 Sep 5;61(36):e202206848. doi: 10.1002/anie.202206848. Epub 2022 Aug 3. Angew Chem Int Ed Engl. 2022. PMID: 35674679 Free PMC article.
-
Catalytic C-H bond amination from high-spin iron imido complexes.J Am Chem Soc. 2011 Apr 6;133(13):4917-23. doi: 10.1021/ja110066j. Epub 2011 Mar 15. J Am Chem Soc. 2011. PMID: 21405138
-
High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving catalysis.Dalton Trans. 2011 Apr 14;40(14):3435-44. doi: 10.1039/c0dt01341b. Epub 2011 Jan 31. Dalton Trans. 2011. PMID: 21279237 Review.
-
Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer.Acc Chem Res. 2021 Mar 2;54(5):1209-1225. doi: 10.1021/acs.accounts.0c00591. Epub 2021 Jan 25. Acc Chem Res. 2021. PMID: 33491448 Free PMC article. Review.
Cited by
-
Copper and Silver Trispyrazolylborate-Phosphinoazide Complexes: Synthesis, Characterization, and Nitrene Generation.Inorg Chem. 2025 Jan 13;64(1):151-157. doi: 10.1021/acs.inorgchem.4c04397. Epub 2025 Jan 2. Inorg Chem. 2025. PMID: 39745493 Free PMC article.
-
Stabilization of a high-spin three-coordinate Fe(iii) imidyl complex by radical delocalization.Chem Sci. 2022 Jul 21;13(33):9637-9643. doi: 10.1039/d2sc02699f. eCollection 2022 Aug 24. Chem Sci. 2022. PMID: 36091897 Free PMC article.
References
-
- Müller P. Fruit C. Chem. Rev. 2003;103:2905–2920. doi: 10.1021/cr020043t. - DOI - PubMed
- Zhang L. Deng L. Chin. Sci. Bull. 2012;57:2352–2360. doi: 10.1007/s11434-012-5151-x. - DOI
- Park Y. Kim Y. Chang S. Chem. Rev. 2017;117:9247–9301. doi: 10.1021/acs.chemrev.6b00644. - DOI - PubMed
- Liu Y. You T. Wang H.-X. Tang Z. Zhou C.-Y. Che C.-M. Chem. Soc. Rev. 2020;49:5310–5358. doi: 10.1039/D0CS00340A. - DOI - PubMed
-
- Strähle J. Angew. Chem. 1989;101:957. doi: 10.1002/ange.19891010728. - DOI
- Kuijpers P. F. van der Vlugt J. I. Schneider S. de Bruin B. Chem.–Eur. J. 2017;23:13819–13829. doi: 10.1002/chem.201702537. - DOI - PMC - PubMed
- Berry J. F. Comments Inorg. Chem. 2009;30:28–66. doi: 10.1080/02603590902768875. - DOI
-
- Ray K. Heims F. Pfaff F. F. Eur. J. Inorg. Chem. 2013;2013:3784–3807. doi: 10.1002/ejic.201300223. - DOI
-
- Grünwald A. Anjana S. S. Munz D. Eur. J. Inorg. Chem. 2021;2021:4147–4166. doi: 10.1002/ejic.202100410. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

