Cobalt-catalyzed chemoselective dehydrogenation through radical translocation under visible light
- PMID: 35865906
- PMCID: PMC9258329
- DOI: 10.1039/d2sc02291e
Cobalt-catalyzed chemoselective dehydrogenation through radical translocation under visible light
Abstract
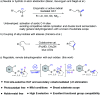
The transformations that allow the direct removal of hydrogen from their corresponding saturated counterparts by the dehydrogenative strategy are a dream reaction that has remained largely underexplored. In this report, a straightforward and robust cobaloxime-catalyzed photochemical dehydrogenation strategy via intramolecular HAT is described for the first time. The reaction proceeds through an intramolecular radical translocation followed by the cobalt assisted dehydrogenation without needing any other external photosensitizers, noble-metals or oxidants. With this approach, a series of valuable unsaturated compounds such as α,β-unsaturated amides, enamides and allylic and homoallylic sulfonamides were obtained in moderate to excellent yields with good chemo- and regioselectivities, and the synthetic versatility was demonstrated by a range of transformations. And mechanistic studies of the method are discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Chemoselective α,β-Dehydrogenation of Saturated Amides.Angew Chem Int Ed Engl. 2019 Jan 8;58(2):447-451. doi: 10.1002/anie.201808794. Epub 2018 Dec 7. Angew Chem Int Ed Engl. 2019. PMID: 30332524 Free PMC article.
-
Site-Selective Acceptorless Dehydrogenation of Aliphatics Enabled by Organophotoredox/Cobalt Dual Catalysis.J Am Chem Soc. 2021 Oct 13;143(40):16470-16485. doi: 10.1021/jacs.1c05479. Epub 2021 Sep 30. J Am Chem Soc. 2021. PMID: 34592106
-
Chemoselective Dehydrogenation and Hetero-Arylation of Amides via Radical Translocation Enabled by Photoexcited Triplet Ketone Catalysis.J Org Chem. 2024 Dec 6;89(23):17818-17823. doi: 10.1021/acs.joc.4c02060. Epub 2024 Nov 16. J Org Chem. 2024. PMID: 39548986
-
Dehydrogenative Pd and Ni Catalysis for Total Synthesis.Acc Chem Res. 2021 Mar 2;54(5):1118-1130. doi: 10.1021/acs.accounts.0c00787. Epub 2021 Feb 16. Acc Chem Res. 2021. PMID: 33592147 Free PMC article. Review.
-
Biocatalytic Hydrogen-Borrowing Cascade in Organic Synthesis.JACS Au. 2024 Mar 12;4(3):877-892. doi: 10.1021/jacsau.4c00026. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559715 Free PMC article. Review.
Cited by
-
Biomimetic Catalytic Remote Desaturation of Aliphatic Alcohols.Org Lett. 2025 Jan 10;27(1):30-35. doi: 10.1021/acs.orglett.4c03623. Epub 2024 Dec 23. Org Lett. 2025. PMID: 39714251 Free PMC article.
-
Catalysis in the Excited State: Bringing Innate Transition Metal Photochemistry into Play.ACS Catal. 2025 Mar 5;15(6):4665-4680. doi: 10.1021/acscatal.4c07962. eCollection 2025 Mar 21. ACS Catal. 2025. PMID: 40144674 Free PMC article. Review.
-
Catalytic dehydrogenative synthesis of α,β-unsaturated secondary amides without external oxidants.Chem Sci. 2024 Aug 23;15(37):15385-90. doi: 10.1039/d4sc04419c. Online ahead of print. Chem Sci. 2024. PMID: 39246373 Free PMC article.
-
Comprehensive Mechanistic Analysis of Palladium- and Nickel-Catalyzed α,β-Dehydrogenation of Carbonyls via Organozinc Intermediates.J Org Chem. 2024 Mar 1;89(5):3123-3132. doi: 10.1021/acs.joc.3c02572. Epub 2024 Feb 20. J Org Chem. 2024. PMID: 38377547 Free PMC article.
-
Cobalt catalyst with exclusive metal-centered chirality for asymmetric photocatalysis.Nat Commun. 2025 Jul 18;16(1):6635. doi: 10.1038/s41467-025-61727-9. Nat Commun. 2025. PMID: 40681516 Free PMC article.
References
-
- Weissermel K. and Arpe H.-J., Olefins, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 2008, p. 59
-
- Breslow R. Snider B. B. Corcoran R. J. J. Am. Chem. Soc. 1974;96:6792–6794. doi: 10.1021/ja00828a059. - DOI - PubMed
- Voica A.-F. Mendoza A. Gutekunst W. R. Fraga J. O. Baran P. S. Nat. Chem. 2012;4:629–635. doi: 10.1038/nchem.1385. - DOI - PMC - PubMed
- Parasram M. Chuentragool P. Sarkar D. Gevorgyan V. J. Am. Chem. Soc. 2016;138:6340–6343. doi: 10.1021/jacs.6b01628. - DOI - PMC - PubMed
- Parasram M. Chuentragool P. Wang Y. Shi Y. Gevorgyan V. J. Am. Chem. Soc. 2017;139:14857–14860. doi: 10.1021/jacs.7b08459. - DOI - PMC - PubMed
- Chuentragool P. Parasram M. Shi Y. Gevorgyan V. J. Am. Chem. Soc. 2018;140:2465–2468. doi: 10.1021/jacs.8b00488. - DOI - PMC - PubMed
- Xia Y. Jana K. Studer A. Chem.–Eur. J. 2021;27:16621–16625. doi: 10.1002/chem.202103509. - DOI - PMC - PubMed
- Stateman L. M. Dare R. M. Paneque A. N. Nagib D. A. Chem. 2022;8:210–224. doi: 10.1016/j.chempr.2021.10.022. - DOI - PMC - PubMed
-
- Zhou M.-J. Zhang L. Liu G. Xu C. Huang Z. J. Am. Chem. Soc. 2021;143:16470–16485. doi: 10.1021/jacs.1c05479. - DOI - PubMed
- West J. G. Huang D. Sorensen E. J. Nat. Commun. 2015;6:10093–10099. doi: 10.1038/ncomms10093. - DOI - PMC - PubMed
- Huang L. Bismuto A. Rath S. A. Trapp N. Morandi B. Angew. Chem., Int. Ed. 2021;60:7290–7296. doi: 10.1002/anie.202015837. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources