Clemastine Promotes Differentiation of Oligodendrocyte Progenitor Cells Through the Activation of ERK1/2 via Muscarinic Receptors After Spinal Cord Injury
- PMID: 35865954
- PMCID: PMC9294397
- DOI: 10.3389/fphar.2022.914153
Clemastine Promotes Differentiation of Oligodendrocyte Progenitor Cells Through the Activation of ERK1/2 via Muscarinic Receptors After Spinal Cord Injury
Abstract
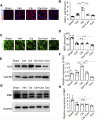
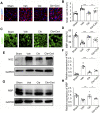
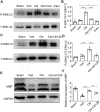
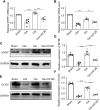
The recovery of spinal cord injury (SCI) is closely associated with the obstruction of oligodendrocyte progenitor cell (OPC) differentiation, which ultimately induces the inability to generate newly formed myelin. To address the concern, drug-based methods may be the most practical and feasible way, possibly applying to clinical therapies for patients with SCI. In our previous study, we found that clemastine treatment preserves myelin integrity, decreases the loss of axons, and improves functional recovery in the SCI model. Clemastine acts as an antagonist of the muscarinic acetylcholine receptor (muscarinic receptor, MR) identified from a string of anti-muscarinic drugs that can enhance oligodendrocyte differentiation and myelin wrapping. However, the effects of clemastine on OPC differentiation through MRs in SCI and the underlying mechanism remain unclear. To explore the possibility, a rat model of SCI was established. To investigate if clemastine could promote the differentiation of OPCs in SCI via MR, the expressions of OPC and mature OL were detected at 7 days post injury (dpi) or at 14 dpi. The significant effect of clemastine on encouraging OPC differentiation was revealed at 14 dpi rather than 7 dpi. Under pre-treatment with the MR agonist cevimeline, the positive role of clemastine on OPC differentiation was partially disrupted. Further studies indicated that clemastine increased the phosphorylation level of extracellular signal-regulated kinase 1/2 (p-ERK1/2) and the expressions of transcription factors, Myrf and Olig2. To determine the relationship among clemastine, ERK1/2 signaling, specified transcription factors, and OPC differentiation, the ERK1/2 signaling was disturbed by U0126. The inhibition of ERK1/2 in SCI rats treated with clemastine decreased the expressions of p-ERK 1/2, Myrf, Olig2, and mature OLs, suggesting that ERK1/2 is required for clemastine on promoting OPC differentiation and that specified transcription factors may be affected by the activity of ERK1/2. Moreover, the impact of clemastine on modulating the level of p-ERK 1/2 was restricted following cevimeline pre-injecting, which provides further evidence that the role of clemastine was mediated by MRs. Altogether, our data demonstrated that clemastine, mediated by MRs, promotes OPC differentiation under the enhancement of Myrf and Olig2 by activating ERK1/2 signaling and suggests a novel therapeutic prospect for SCI recovery.
Keywords: ERK1/2; clemastine; muscarinic receptor; myelin regulatory factor; oligodendrocyte progenitor cells; oligodendrocyte transcription factor 2.
Copyright © 2022 Tong, Deng, Du, Zhou, Liao and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

