Antibody-Targeted TNFRSF Activation for Cancer Immunotherapy: The Role of FcγRIIB Cross-Linking
- PMID: 35865955
- PMCID: PMC9295861
- DOI: 10.3389/fphar.2022.924197
Antibody-Targeted TNFRSF Activation for Cancer Immunotherapy: The Role of FcγRIIB Cross-Linking
Abstract
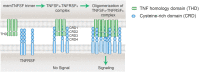
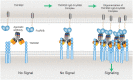
Co-stimulation signaling in various types of immune cells modulates immune responses in physiology and disease. Tumor necrosis factor receptor superfamily (TNFRSF) members such as CD40, OX40 and CD137/4-1BB are expressed on myeloid cells and/or lymphocytes, and they regulate antigen presentation and adaptive immune activities. TNFRSF agonistic antibodies have been evaluated extensively in preclinical models, and the robust antitumor immune responses and efficacy have encouraged continued clinical investigations for the last two decades. However, balancing the toxicities and efficacy of TNFRSF agonistic antibodies remains a major challenge in the clinical development. Insights into the co-stimulation signaling biology, antibody structural roles and their functionality in immuno-oncology are guiding new advancement of this field. Leveraging the interactions between antibodies and the inhibitory Fc receptor FcγRIIB to optimize co-stimulation agonistic activities dependent on FcγRIIB cross-linking selectively in tumor microenvironment represents the current frontier, which also includes cross-linking through tumor antigen binding with bispecific antibodies. In this review, we will summarize the immunological roles of TNFRSF members and current clinical studies of TNFRSF agonistic antibodies. We will also cover the contribution of different IgG structure domains to these agonistic activities, with a focus on the role of FcγRIIB in TNFRSF cross-linking and clustering bridged by agonistic antibodies. We will review and discuss several Fc-engineering approaches to optimize Fc binding ability to FcγRIIB in the context of proper Fab and the epitope, including a cross-linking antibody (xLinkAb) model and its application in developing TNFRSF agonistic antibodies with improved efficacy and safety for cancer immunotherapy.
Keywords: CD137 (4-1BB); CD40; FcγRIIB; TNFRSF; agonist; cancer; cross-linking; immunotherapy.
Copyright © 2022 Liu, Wu, Ye, Cai, Zhuang and Wang.
Conflict of interest statement
LL, YW and JW are employees of Lyvgen Biopharma, a private biotech company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

