Accuracy of two dosimetry software programs for 177Lu radiopharmaceutical therapy using voxel-based patient-specific phantoms
- PMID: 35865988
- PMCID: PMC9293745
- DOI: 10.1016/j.heliyon.2022.e09830
Accuracy of two dosimetry software programs for 177Lu radiopharmaceutical therapy using voxel-based patient-specific phantoms
Abstract
Purpose: Virtual dosimetry using voxel-based patient-specific phantoms and Monte Carlo (MC) simulations offer the advantage of having a gold standard against which absorbed doses may be benchmarked to establish the dosimetry accuracy. Furthermore, these reference values assist in investigating the accuracy of the absorbed dose methodologies from different software programs. Therefore, this study aimed to compare the accuracy of the absorbed doses computed using LundADose and OLINDA/EXM 1.0.
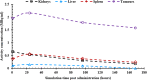
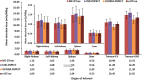
Methods: The accuracy was based on 177Lu-DOTATATE distributions of three voxel-based phantoms. SPECT projection images were simulated for 1, 24, 96, and 168 h post-administration and reconstructed with LundADose using 3D OS-EM reconstruction. Mono-exponential curves were fitted to the bio-kinetic data for the kidneys, liver, spleen, and tumours resulting in SPECT time-integrated activity (SPECT-TIA). The SPECT-TIA were used to compute mean absorbed doses using LundADose (LND-DSPECT) and OLINDA (OLINDA-DSPECT) for the organs. Pre-defined true activity images, were used to obtain TRUE-TIA and, together with full MC simulations, computed the true doses (MC-DTrue). The dosimetry accuracy was assessed by comparing LND-DSPECT and OLINDA-DSPECT to MC-DTrue.
Results: Overall, the results presented an overestimation of the mean absorbed dose by LND-DSPECT compared to the MC-DTrue with a dosimetry accuracy ≤6.6%. This was attributed to spill-out activity from the reconstructed LND-DSPECT, resulting in a higher dose contribution than the MC-DTrue. There was a general underestimation (<8.1%) of OLINDA-DSPECT compared to MC-DTrue attributed to the geometrical difference in shape between the voxel-based phantoms and the OLINDA models. Furthermore, OLINDA-DSPECT considers self-doses while MC-DTrue reflects self-doses plus cross-doses.
Conclusion: The better than 10% accuracy suggests that the mean dose values obtained with LND-DSPECT and OLINDA-DSPECT approximate the true values. The mean absorbed doses of the two software programs, and the gold standard were comparable. This work shall be of use for optimising 177Lu dosimetry for clinical applications.
Keywords: 177Lu-DOTATATE; Accuracy; Dosimetry; LundADose; Monte Carlo; OLINDA; Patient-specific phantom; Radiopharmaceutical therapy; SPECT; Voxel-based phantom.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Linear Boltzmann equation solver for voxel-level dosimetry in radiopharmaceutical therapy: Comparison with Monte Carlo and kernel convolution.Med Phys. 2024 Aug;51(8):5604-5617. doi: 10.1002/mp.16996. Epub 2024 Mar 4. Med Phys. 2024. PMID: 38436493 Free PMC article.
-
Personalized dosimetry of177Lu-DOTATATE: a comparison of organ- and voxel-level approaches using open-access images.Biomed Phys Eng Express. 2021 Aug 4;7(5):10.1088/2057-1976/ac1550. doi: 10.1088/2057-1976/ac1550. Biomed Phys Eng Express. 2021. PMID: 34271565 Free PMC article.
-
Deep transformer-based personalized dosimetry from SPECT/CT images: a hybrid approach for [177Lu]Lu-DOTATATE radiopharmaceutical therapy.Eur J Nucl Med Mol Imaging. 2024 May;51(6):1516-1529. doi: 10.1007/s00259-024-06618-9. Epub 2024 Jan 25. Eur J Nucl Med Mol Imaging. 2024. PMID: 38267686 Free PMC article.
-
Preclinical Voxel-Based Dosimetry in Theranostics: a Review.Nucl Med Mol Imaging. 2020 Apr;54(2):86-97. doi: 10.1007/s13139-020-00640-z. Epub 2020 Apr 19. Nucl Med Mol Imaging. 2020. PMID: 32377260 Free PMC article. Review.
-
Activity quantification and dosimetry in radiopharmaceutical therapy with reference to 177Lutetium.Front Nucl Med. 2024 Mar 28;4:1355912. doi: 10.3389/fnume.2024.1355912. eCollection 2024. Front Nucl Med. 2024. PMID: 39355215 Free PMC article. Review.
Cited by
-
Predicting the spatio-temporal response of recurrent glioblastoma treated with rhenium-186 labelled nanoliposomes.Brain Multiphys. 2023 Dec;5:100084. doi: 10.1016/j.brain.2023.100084. Epub 2023 Oct 29. Brain Multiphys. 2023. PMID: 38187909 Free PMC article.
-
Evaluating auto-contouring accuracy in reduced CT dose images for radiopharmaceutical therapies: Denoising and evaluation of 177Lu DOTATATE therapy dataset.J Appl Clin Med Phys. 2025 Apr;26(4):e70066. doi: 10.1002/acm2.70066. Epub 2025 Mar 2. J Appl Clin Med Phys. 2025. PMID: 40025651 Free PMC article.
-
A review of 177Lu dosimetry workflows: how to reduce the imaging workloads?EJNMMI Phys. 2024 Jul 18;11(1):65. doi: 10.1186/s40658-024-00658-8. EJNMMI Phys. 2024. PMID: 39023648 Free PMC article. Review.
-
Quantitative whole-body dynamic planar scintigraphy in mice with 99mTc and 161Tb.EJNMMI Phys. 2025 Jul 1;12(1):61. doi: 10.1186/s40658-025-00775-y. EJNMMI Phys. 2025. PMID: 40591183 Free PMC article.
-
Feasibility of individual dosimetry using RT-PHITS for patients with SPECT/CT imaging after 177Lu-DOTATATE peptide receptor radionuclide therapy.Phys Eng Sci Med. 2025 Jun;48(2):949-957. doi: 10.1007/s13246-025-01551-z. Epub 2025 May 6. Phys Eng Sci Med. 2025. PMID: 40327234
References
-
- Wahl R.L., Ahuja S., Clarke B. Current landscape of radiopharmaceutical therapies: SNMMI therapy task force survey. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021 May 10;62(5):11N–16N. - PubMed
-
- Bodei L., Cremonesi M., Ferrari M., Pacifici M., Grana C.M., Bartolomei M., et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur. J. Nucl. Med. Mol. Imag. 2008 Oct;35(10):1847–1856. - PubMed
-
- Kendi A.T., Halfdanarson T.R., Packard A., Dundar A., Subramaniam R.M. Therapy with 177Lu-DOTATATE: clinical implementation and impact on care of patients with neuroendocrine tumors. Am. J. Roentgenol. 2019 Aug 1;213(2):309–317. - PubMed
-
- Kwekkeboom D.J., de Herder W.W., Kam B.L., van Eijck C.H., van Essen M., Kooij P.P., et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008 May 1;26(13):2124–2130. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

