Effect of the Human Amniotic Membrane on the Umbilical Vein Endothelial Cells of Gestational Diabetic Mothers: New Insight on Inflammation and Angiogenesis
- PMID: 35866032
- PMCID: PMC9294233
- DOI: 10.3389/fbioe.2022.854845
Effect of the Human Amniotic Membrane on the Umbilical Vein Endothelial Cells of Gestational Diabetic Mothers: New Insight on Inflammation and Angiogenesis
Abstract
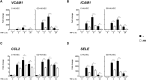
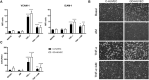
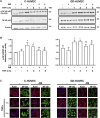
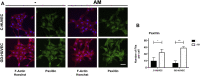
One of the most relevant diabetes complications is impaired wound healing, mainly characterized by reduced peripheral blood flow and diminished neovascularization together with increased inflammation and oxidative stress. Unfortunately, effective therapies are currently lacking. Recently, the amniotic membrane (AM) has shown promising results in wound management. Here, the potential role of AM on endothelial cells isolated from the umbilical cord vein of gestational diabetes-affected women (GD-HUVECs), has been investigated. Indeed, GD-HUVECs in vivo exposed to chronic hyperglycemia during pregnancy compared to control cells (C-HUVECs) have shown molecular modifications of cellular homeostasis ultimately impacting oxidative and nitro-oxidative stress, inflammatory phenotype, nitric oxide (NO) synthesis, and bioavailability, thus representing a useful model for studying the mechanisms potentially supporting the role of AM in chronic non-healing wounds. In this study, the anti-inflammatory properties of AM have been assessed using a monocyte-endothelium interaction assay in cells pre-stimulated with tumor necrosis factor-α (TNF-α) and through vascular adhesion molecule expression and membrane exposure, together with the AM impact on the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-kB) pathway and NO bioavailability. Moreover, GD-HUVEC migration and tube formation ability were evaluated in the presence of AM. The results showed that AM significantly reduced TNF-α-stimulated monocyte-endothelium interaction and the membrane exposure of the endothelial vascular and intracellular adhesion molecules (VCAM-1 and ICAM-1, respectively) in both C- and GD-HUVECs. Strikingly, AM treatment significantly improved vessel formation in GD-HUVECs and cell migration in both C- and GD-HUVECs. These collective results suggest that AM positively affects various critical pathways in inflammation and angiogenesis, thus providing further validation for ongoing clinical trials in diabetic foot ulcers.
Keywords: HUVECs; amniotic membrane (AM); angiogenesis; diabetes; inflammation; wound healing.
Copyright © 2022 Pipino, Bernabé-García, Cappellacci, Stelling-Férez, Di Tomo, Santalucia, Navalón, Pandolfi and Nicolás.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Centella asiatica and lipoic acid, or a combination thereof, inhibit monocyte adhesion to endothelial cells from umbilical cords of gestational diabetic women.Nutr Metab Cardiovasc Dis. 2015 Jul;25(7):659-66. doi: 10.1016/j.numecd.2015.04.002. Epub 2015 Apr 22. Nutr Metab Cardiovasc Dis. 2015. PMID: 26026207
-
Oleanolic acid rescues critical features of umbilical vein endothelial cells permanently affected by hyperglycemia.Front Endocrinol (Lausanne). 2023 Dec 13;14:1308606. doi: 10.3389/fendo.2023.1308606. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38192424 Free PMC article.
-
Features of endothelial dysfunction in umbilical cord vessels of women with gestational diabetes.Nutr Metab Cardiovasc Dis. 2014 Dec;24(12):1337-45. doi: 10.1016/j.numecd.2014.06.005. Nutr Metab Cardiovasc Dis. 2014. PMID: 25438716
-
Anti-inflammatory Role of Carotenoids in Endothelial Cells Derived from Umbilical Cord of Women Affected by Gestational Diabetes Mellitus.Oxid Med Cell Longev. 2019 Jan 30;2019:8184656. doi: 10.1155/2019/8184656. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30918580 Free PMC article.
-
Myoinositol Reduces Inflammation and Oxidative Stress in Human Endothelial Cells Exposed In Vivo to Chronic Hyperglycemia.Nutrients. 2021 Jun 27;13(7):2210. doi: 10.3390/nu13072210. Nutrients. 2021. PMID: 34199095 Free PMC article.
Cited by
-
Amniotic Membrane Restores Chronic Wound Features to Normal in a Keratinocyte TGF-β-Chronified Cell Model.Int J Mol Sci. 2023 Mar 25;24(7):6210. doi: 10.3390/ijms24076210. Int J Mol Sci. 2023. PMID: 37047181 Free PMC article.
-
Recommendations from the COST action CA17116 (SPRINT) for the standardization of perinatal derivative preparation and in vitro testing.Front Bioeng Biotechnol. 2023 Nov 14;11:1258753. doi: 10.3389/fbioe.2023.1258753. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38033821 Free PMC article.
-
Cardioprotective Peptides from Dry-Cured Ham in Primary Endothelial Cells and Human Plasma: An Omics Approach.Antioxidants (Basel). 2025 Jun 24;14(7):772. doi: 10.3390/antiox14070772. Antioxidants (Basel). 2025. PMID: 40722877 Free PMC article.
-
State of the Art and New Trends from the Second International StemNet Meeting.Int J Mol Sci. 2024 Feb 13;25(4):2221. doi: 10.3390/ijms25042221. Int J Mol Sci. 2024. PMID: 38396899 Free PMC article.
-
Exploring the influence of growth factors in diabetic foot: A comprehensive bibliometric analysis.Medicine (Baltimore). 2025 Aug 1;104(31):e42716. doi: 10.1097/MD.0000000000042716. Medicine (Baltimore). 2025. PMID: 40760573 Free PMC article.
References
-
- Alcaraz A., Mrowiec A., Insausti C. L., Bernabé-García Á., García-Vizcaíno E. M., López-Martínez M. C., et al. (2015). Amniotic Membrane Modifies the Genetic Program Induced by TGFß, Stimulating Keratinocyte Proliferation and Migration in Chronic Wounds. PLoS ONE 10 (8), e0135324. 10.1371/journal.pone.0135324 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

