New wide-stability four-ring azo/ester/Schiff base liquid crystals: synthesis, mesomorphic, photophysical, and DFT approaches
- PMID: 35866044
- PMCID: PMC9261499
- DOI: 10.1039/c9ra10499b
New wide-stability four-ring azo/ester/Schiff base liquid crystals: synthesis, mesomorphic, photophysical, and DFT approaches
Abstract
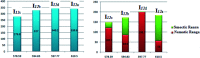
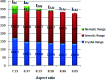
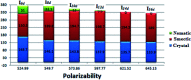
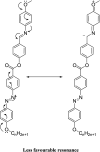
New four-groups-based azo/ester/Schiff base liquid crystals, ((4-substitutedphenylimino)methyl)phenyl 4-[2-(4-alkoxyhenyl)diazenyl]benzoate, In a-d, were synthesized and analyzed for their mesomorphic stability and optical activity. In these compounds, a terminal alkoxy group of variable chain length from n = 6 to n = 16 carbons is attached to the end of a phenylazo benzoate moiety and the other end of the molecules is connected to a different polar compact substituent X (CH3O, CH3, H, and Cl). FT-IR, 1H NMR, mass spectroscopy and elemental analysis were carried out for molecular structure confirmation of the prepared compounds. The mesomorphic properties were confirmed using a combination of differential scanning calorimetry (DSC) and polarized light microscopy (PLM). The photophysical property was studied by UV-vis spectroscopy. All the prepared homologous series exhibited high thermal stability with a wide-temperature mesomorphic range. The thermal and geometrical parameters of the investigated compounds were estimated by density functional theory (DFT). The results revealed that all the compounds were not completely planar with a relatively high twisting moiety at the CH[double bond, length as m-dash]N part and their twist angles were affected by the electronic nature of the attached X group. Moreover, the calculated quantum chemical parameters as determined by the DFT approach of the investigated compounds were related to the experimentally determined values of the mesophase thermal stability (T c) and mesophase temperature ranges (ΔT SmA and ΔT N) as well as the type of the mesophase.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures











References
-
- Imrie C. T. Henderson P. A. Yeap G.-Y. Liq. Cryst. 2009;36:755–777. doi: 10.1080/02678290903157455. - DOI
-
- Yeap G.-Y. Lee H.-C. Mahmood W. A. K. Imrie C. T. Takeuchi D. Osakada K. Phase Transitions. 2011;84:29–37. doi: 10.1080/01411594.2010.513613. - DOI
-
- Yeap G.-Y. Osman F. Imrie C. T. Liq. Cryst. 2015;42:543–554. doi: 10.1080/02678292.2015.1004843. - DOI
-
- Yeap G.-Y. Hng T.-C. Yeap S.-Y. Gorecka E. Ito M. M. Ueno K. Okamoto M. Mahmood W. A. K. Imrie C. T. Liq. Cryst. 2009;36:1431–1441. doi: 10.1080/02678290903271504. - DOI
-
- Pramanik A. Das M. K. Das B. Żurowska M. Dąbrowski R. Liq. Cryst. 2015;42:412–421. doi: 10.1080/02678292.2014.996792. - DOI
LinkOut - more resources
Full Text Sources

