The Cortical Bone Metabolome of C57BL/6J Mice Is Sexually Dimorphic
- PMID: 35866150
- PMCID: PMC9289981
- DOI: 10.1002/jbm4.10654
The Cortical Bone Metabolome of C57BL/6J Mice Is Sexually Dimorphic
Abstract
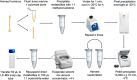
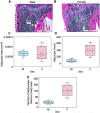
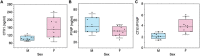
Cortical bone quality, which is sexually dimorphic, depends on bone turnover and therefore on the activities of remodeling bone cells. However, sex differences in cortical bone metabolism are not yet defined. Adding to the uncertainty about cortical bone metabolism, the metabolomes of whole bone, isolated cortical bone without marrow, and bone marrow have not been compared. We hypothesized that the metabolome of isolated cortical bone would be distinct from that of bone marrow and would reveal sex differences. Metabolite profiles from liquid chromatography-mass spectrometry (LC-MS) of whole bone, isolated cortical bone, and bone marrow were generated from humeri from 20-week-old female C57Bl/6J mice. The cortical bone metabolomes were then compared for 20-week-old female and male C57Bl/6J mice. Femurs from male and female mice were evaluated for flexural material properties and were then categorized into bone strength groups. The metabolome of isolated cortical bone was distinct from both whole bone and bone marrow. We also found sex differences in the isolated cortical bone metabolome. Based on metabolite pathway analysis, females had higher lipid metabolism, and males had higher amino acid metabolism. High-strength bones, regardless of sex, had greater tryptophan and purine metabolism. For males, high-strength bones had upregulated nucleotide metabolism, whereas lower-strength bones had greater pentose phosphate pathway metabolism. Because the higher-strength groups (females compared with males, high-strength males compared with lower-strength males) had higher serum type I collagen cross-linked C-telopeptide (CTX1)/procollagen type 1 N propeptide (P1NP), we estimate that the metabolomic signature of bone strength in our study at least partially reflects differences in bone turnover. These data provide novel insight into bone bioenergetics and the sexual dimorphic nature of bone material properties in C57Bl/6 mice. © 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: BONE QUALITY; METABOLISM; METABOLOMICS; SEX DIFFERENCES.
© 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Conflict of interest statement
The authors have no conflicts of interest to disclose. Dr. June owns stock in Beartooth Biotech which was not involved in this study.
Figures



References
-
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age‐related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22(8):1197‐1207. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
