Pregnancy Vitamin D Supplementation and Childhood Bone Mass at Age 4 Years: Findings From the Maternal Vitamin D Osteoporosis Study (MAVIDOS) Randomized Controlled Trial
- PMID: 35866154
- PMCID: PMC9289979
- DOI: 10.1002/jbm4.10651
Pregnancy Vitamin D Supplementation and Childhood Bone Mass at Age 4 Years: Findings From the Maternal Vitamin D Osteoporosis Study (MAVIDOS) Randomized Controlled Trial
Abstract
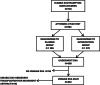
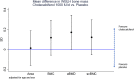
In the Maternal Vitamin D Osteoporosis Study (MAVIDOS) randomized trial, vitamin D supplementation in pregnancy did not lead to greater neonatal bone mass across the trial as a whole, but, in a prespecified secondary analysis by season of birth, led to greater neonatal bone mass among winter-born babies. Demonstrating persistence of this effect into childhood would increase confidence in a long-term benefit of this intervention. We investigated whether antenatal vitamin D supplementation increases offspring bone mineralization in early childhood in a prespecified, single-center follow-up of a double-blinded, multicenter, randomized controlled clinical trial based in the UK (MAVIDOS). A total of 1123 women in early pregnancy with a baseline 25-hydroxyvitamin D level 25-100 nmol/L from three research centers (2008-2014) were randomized to 1000 IU/d cholecalciferol or matched placebo from 14 weeks of gestation to delivery. Offspring born at the Southampton, UK research center were assessed at age 4 years (2013-2018). Anthropometry and dual-energy X-ray absorptiometry (DXA) were performed (yielding whole body less head [WBLH] bone mineral content [BMC], areal bone mineral density [aBMD], bone area [BA], and body composition). Of 723 children, 564 (78.0%) children attended the 4-year visit, 452 of whom had a useable DXA. Maternal vitamin D supplementation led to greater WBLH aBMD in the children compared with placebo (mean [95% confidence interval {CI}]: supplemented group: 0.477 (95% CI, 0.472-0.481) g/cm2; placebo group: 0.470 (95% CI, 0.466-0.475) g/cm2, p = 0.048). Associations were consistent for BMC and lean mass, and in age- and sex-adjusted models. Effects were observed across the whole cohort irrespective of season of birth. Maternal-child interactions were observed, with a greater effect size among children with low milk intake and low levels of physical activity. Child weight, height, and body mass index (BMI) were similar by maternal randomization group. These findings suggest a sustained beneficial effect of maternal vitamin D supplementation in pregnancy on offspring aBMD at age 4 years, but will require replication in other trials. © 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: CLINICAL TRIALS; DXA; FRACTURE PREVENTION; NUTRITION; OSTEOPOROSIS.
© 2022 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Conflict of interest statement
EMC reports honoraria/travel support from Eli Lilly, Pfizer, and UCB outside the submitted work. NJB reports remuneration from Internis Pharmaceuticals Ltd, outside the submitted work. ATP reports grants from Versus Arthritis, Medical Research Council, National Institute for Health Research, Bupa Foundation, BBSRC, and EU outside the submitted work. KMG reports reimbursement for speaking at Nestle Nutrition Institute conferences, grants from Abbott Nutrition & Nestec, outside the submitted work; in addition, KMG has a patent Phenotype Prediction pending, a patent Predictive Use of CpG Methylation pending, and a patent Maternal Nutrition Composition pending, not directly related to this work. MKJ reports personal fees from Stirling Anglia, Consilient Health and Internis, outside the submitted work. RE reports grants from Amgen, grants and personal fees from IDS, grants from Alexion, grants and personal fees from Roche, personal fees from GSK Nutrition, personal fees from Mereo, personal fees from Sandoz, grants and personal fees from Nittobo, personal fees from AbbVie, personal fees from Samsung, personal fees from Haoma Medica, personal fees from Elsevier, personal fees from CL Bio, personal fees from FNIH, personal fees from Viking, personal fees from UCSF, personal fees from Biocon, from Lyramid, outside the submitted work. CC reports personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda, outside the submitted work. NCH reports personal fees, consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Consilient Healthcare, Kyowa Kirin and Internis Pharma, outside the submitted work. RJM, SD, SRC, JSGK, SHK, RF, SVG, IS, AP, and HMI have nothing to disclose. Of the MAVIDOS group: N. Arden has received honoraria, held advisory board positions (which involved receipt of fees), and received consortium research grants, respectively, from: Merck, grants from Roche, personal fees from Smith & Nephew, Nicox, Flexion, grants from Bioiberica, Novartis, and personal fees from Bioventus and Freshfields, outside the submitted work. M.Z. Mughal has received lecture fees from Abbott Nutrition & Thornton & Ross, outside the submitted work. A. Carr, M. Clynes, E. Dennison, D. Reid, and S. Woolford have nothing to disclose.
Figures

References
-
- Harvey N, Dennison E, Cooper C. Osteoporosis: a lifecourse approach. J Bone Miner Res. 2014;29(9):1917‐1925. - PubMed
-
- Harvey NC, Moon RJ, Cooper C. Vitamin D in early life: from observation to intervention. In Harvey NC, Cooper C, eds. Osteoporosis: A Lifecourse Epidemiology Approach to Skeletal Health. Boca Raton: CRC Press; 2018. pp 53‐64.
-
- Javaid MK, Crozier SR, Harvey NC, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367(9504):36‐43. - PubMed
-
- Viljakainen HT, Saarnio E, Hytinantti T, et al. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95(4):1749‐1757. - PubMed
Grants and funding
- 17702/VAC_/Versus Arthritis/United Kingdom
- MC_UP_A620_1015/MRC_/Medical Research Council/United Kingdom
- 21231/VAC_/Versus Arthritis/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/2/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- MC_PC_21000/MRC_/Medical Research Council/United Kingdom
- MC_PC_15015/MRC_/Medical Research Council/United Kingdom
- 201222/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_21022/MRC_/Medical Research Council/United Kingdom
- MR/P020941/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_21002/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
