Post stroke intervention trial in fatigue (POSITIF): Randomised multicentre feasibility trial
- PMID: 35866206
- PMCID: PMC9574032
- DOI: 10.1177/02692155221113908
Post stroke intervention trial in fatigue (POSITIF): Randomised multicentre feasibility trial
Abstract
Objective: To test the feasibility of a telephone delivered intervention, informed by cognitive behavioural principles, for post-stroke fatigue, and estimated its effect on fatigue and other outcomes.
Design: Randomised controlled parallel group trial.
Setting: Three Scottish stroke services.
Subjects: Stroke survivors with fatigue three months to two years post-stroke onset.
Interventions: Seven telephone calls (fortnightly then a 'booster session' at 16 weeks) of a manualised intervention, plus information about fatigue, versus information only.
Main measures: Feasibility of trial methods, and collected outcome measures (fatigue, mood, anxiety, social participation, quality of life, return to work) just before randomisation, at the end of treatment (four months after randomisation) and at six months after randomisation.
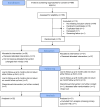
Results: Between October 2018 and January 2020, we invited 886 stroke survivors to participate in postal screening: 188/886 (21%) returned questionnaires and consented, of whom 76/188 (40%) were eligible and returned baseline forms; 64/76 (84%) returned six month follow-up questionnaires. Of the 39 allocated the intervention, 23 (59%) attended at least four sessions. At six months, there were no significant differences between the groups (adjusted mean differences in Fatigue Assessment Scale -0.619 (95% CI -4.9631, 3.694; p = 0.768), the Generalised Anxiety Disorder 7 -0.178 (95% CI -3.823, 3.467, p = 0.92), and the Patient Health Questionnaire -0.247 (95% CI -2.935, 2.442, p = 0.851). There were no between-group differences in quality of life, social participation or return to work.
Conclusion: Patients can be recruited to a trial of this design. These data will inform the design of further trials in post-stroke fatigue.
Keywords: Fatigue; randomized controlled trial; stroke.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
-
- Annoni J-M, Staub F, Bogousslavsky J, et al. Frequency, characterisation and therapies of fatigue after stroke. Neurol Sci 2008; 29: S244–S246. - PubMed
-
- Hinkle JL, Becker KJ, Kim J, et al. On behalf of the American heart association council on cardiovascular and stroke nursing and stroke council poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American heart association. Stroke 2017; 48: e159–e170. - PubMed
-
- Stroke Association. Shaping stroke research to rebuild lives. The Stroke Priority Setting partnership results for investment. June 2021. Item code: A08C19 © Stroke Association 2021.
-
- Duncan F, Greig CA, Dennis MS, et al. An exploratory longitudinal cohort study of associations of fatigue after stroke. Stroke 2015; 46: 1052–1058. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical