Automated Methods for Detecting and Quantitation of Enlarged Perivascular spaces on MRI
- PMID: 35866259
- PMCID: PMC10083963
- DOI: 10.1002/jmri.28369
Automated Methods for Detecting and Quantitation of Enlarged Perivascular spaces on MRI
Abstract
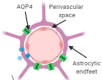
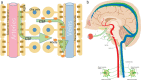
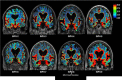
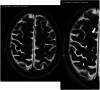
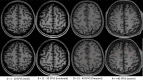
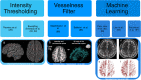
The brain's glymphatic system is a network of intracerebral vessels that function to remove "waste products" such as degraded proteins from the brain. It comprises of the vasculature, perivascular spaces (PVS), and astrocytes. Poor glymphatic function has been implicated in numerous diseases; however, its contribution is still unknown. Efforts have been made to image the glymphatic system to further assess its role in the pathogenesis of different diseases. Numerous imaging modalities have been utilized including two-photon microscopy and contrast-enhanced magnetic resonance imaging (MRI). However, these are associated with limitations for clinical use. PVS form a part of the glymphatic system and can be visualized on standard MRI sequences when enlarged. It is thought that PVS become enlarged secondary to poor glymphatic drainage of metabolites. Thus, quantitating PVS could be a good surrogate marker for glymphatic function. Numerous manual rating scales have been developed to measure the PVS number and size on MRI scans; however, these are associated with many limitations. Instead, automated methods have been created to measure PVS more accurately in different diseases. In this review, we discuss the imaging techniques currently available to visualize the glymphatic system as well as the automated methods currently available to measure PVS, and the strengths and limitations associated with each technique. EVIDENCE LEVEL: 1 TECHNICAL EFFICACY: Stage 1.
Keywords: MRI; Virchow-Robin spaces; brain; glymphatic; perivascular spaces; quantification.
© 2022 The Authors. Journal of Magnetic Resonance Imaging published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

