Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer
- PMID: 35866378
- PMCID: PMC9309650
- DOI: 10.1002/14651858.CD008766.pub3
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer
Abstract
Background: Ovarian cancer is the seventh most frequent cancer diagnosis worldwide, and the eighth leading cause of cancer mortality. Epithelial ovarian cancer is the most common kind, accounting for 90% of cases. First-line therapy for women with epithelial ovarian cancer consists of a combination of cytoreductive surgery and platinum and taxane-based chemotherapy. However, more than 50% of women with epithelial ovarian cancer will experience a relapse and require further chemotherapy and at some point develop resistance to platinum-based drugs. Currently, guidance on the use of most chemotherapy drugs, including taxanes, is unclear for women whose epithelial ovarian cancer has recurred. Paclitaxel, topotecan, pegylated liposomal doxorubicin hydrochloride, trabectedin and gemcitabine are all licensed for use in the UK at the discretion of clinicians, following discussion with the women as to potential adverse effects. Taxanes can be given in once-weekly regimens (at a lower dose) or three-weekly regimens (at a higher dose), which may have differences in the severity of side effects and effectiveness. As relapsed disease suggests incurable disease, it is all the more important to consider side effects and the impact of treatment schedules, as well as quality of life, and not only the life-prolonging effects of treatment.
Objectives: To assess the efficacy and toxicity of different taxane monotherapy regimens for women with recurrent epithelial ovarian, tubal or primary peritoneal cancer.
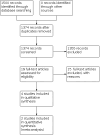
Search methods: We searched CENTRAL, MEDLINE and Embase, up to 22 March 2022. Other related databases and trial registries were searched as well as grey literature and no additional studies were identified. A total of 1500 records were identified.
Selection criteria: We included randomised controlled trials of taxane monotherapy for adult women diagnosed with recurrent epithelial ovarian, tubal or primary peritoneal cancer, previously treated with platinum-based chemotherapy. We included trials comparing two or more taxane monotherapy regimens. Participants could be experiencing their first recurrence of disease or any line of recurrence.
Data collection and analysis: Two review authors screened, independently assessed studies, and extracted data from the included studies. The clinical outcomes we examined were overall survival, response rate, progression-free survival, neurotoxicity, neutropenia, alopecia, and quality of life. We performed statistical analyses using fixed-effect and random-effects models following standard Cochrane methodology. We rated the certainty of evidence according to the GRADE approach.
Main results: Our literature search yielded 1500 records of 1466 studies; no additional studies were identified by searching grey literature or handsearching. We uploaded the search results into Covidence. After the exclusion of 92 duplicates, we screened titles and abstracts of 1374 records. Of these, we identified 24 studies for full-text screening. We included four parallel-group randomised controlled trials (RCTs). All trials were multicentred and conducted in a hospital setting. The studies included 981 eligible participants with recurrent epithelial ovarian cancer, tubal or primary peritoneal cancer with a median age ranging between 56 to 62 years of age. All participants had a WHO (World Health Organization) performance status of between 0 to 2. The proportion of participants with serous histology ranged between 56% to 85%. Participants included women who had platinum-sensitive (71%) and platinum-resistant (29%) relapse. Some participants were taxane pre-treated (5.6%), whilst the majority were taxane-naive (94.4%). No studies were classified as having a high risk of bias for any of the domains in the Cochrane risk of bias tool. We found that there may be little or no difference in overall survival (OS) between weekly paclitaxel and three-weekly paclitaxel, but the evidence is very uncertain (risk ratio (RR) of 0.94, 95% confidence interval (CI) 0.66 to 1.33, two studies, 263 participants, very low-certainty evidence). Similarly, there may be little or no difference in response rate (RR of 1.07, 95% CI 0.78 to 1.48, two studies, 263 participants, very low-certainty evidence) and progression-free survival (PFS) (RR of 0.83, 95% CI 0.46 to 1.52, two studies, 263 participants, very low-certainty evidence) between weekly and three-weekly paclitaxel, but the evidence is very uncertain. We found differences in the chemotherapy-associated adverse events between the weekly and three-weekly paclitaxel regimens. The weekly paclitaxel regimen may result in a reduction in neutropenia (RR 0.51, 95% 0.27 to 0.95, two studies, 260 participants, low-certainty evidence) and alopecia (RR 0.58, 95% CI 0.46 to 0.73, one study, 205 participants, low-certainty evidence). There may be little or no difference in neurotoxicity, but the evidence was very low-certainty and we cannot exclude an effect (RR 0.53, 95% CI 0.19 to 1.45, two studies, 260 participants). When examining the effect of paclitaxel dosage in the three-weekly regimen, the 250 mg/m2 paclitaxel regimen probably causes more neurotoxicity compared to the 175 mg/m2 regimen (RR 0.41, 95% CI 0.21 to 0.80, one study, 330 participants, moderate-certainty evidence). Quality-of-life data were not extractable from any of the included studies.
Authors' conclusions: Fewer people may experience neutropenia when given weekly rather than three-weekly paclitaxel (low-certainty evidence), although it may make little or no difference to the risk of developing neurotoxicity (very low-certainty evidence). This is based on the participants receiving lower doses of drug more often. However, our confidence in this result is low and the true effect may be substantially different from the estimate of the effect. Weekly paclitaxel probably reduces the risk of alopecia, although the rates in both arms were high (46% versus 79%) (low-certainty evidence). A change to weekly from three-weekly chemotherapy could be considered to reduce the likelihood of toxicity, as it may have little or no negative impact on response rate (very low-certainty evidence), PFS (very low-certainty evidence) or OS (very low-certainty evidence). Three-weekly paclitaxel, given at a dose of 175 mg/m2 compared to a higher dose,probably reduces the risk of neurotoxicity.We are moderately confident in this result; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. A change to 175 mg/m2 paclitaxel (from a higher dose), if a three-weekly regimen is used, probably has little or no negative impact on PFS or OS (very low-certainty evidence).
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Aashna Patel: no relevant interests were disclosed. Roshni Kalachand: no relevant interests were disclosed. Steven Buscchots: no relevant interests were disclosed. Ben Doherty: no relevant interests were disclosed. Evangelos Kapros: no relevant interests were disclosed. Denise Lawlor: no relevant interests were disclosed. Neville Hall: no relevant interests were disclosed. Britta Stordal: has declared she is employed by Middlesex University London and has worked on a contract basis for the Research Executive Agency reviewing grants. She has received grant funding from the Health Research Board in support of this review and from the Irish Cancer Society and Bone Cancer Trust in support of other projects. She has also received travel expenses from the National Institute for Health and Care Excellence (NICE) in her role as a specialist committee member.
Figures























Update of
- doi: 10.1002/14651858.CD008766.pub2
References
References to studies included in this review
Eisenhauer 1994 {published data only}
-
- Eisenhauer E, Bacon M, Walsh W, McDaniel C, Canetta R, Onetto N, et al. Long term survivors from a European-Canadian trial of paclitaxel in platinum-pretreated ovarian cancer (OVCA). In: European Cancer Conference; 1997. 1997:Abstract 532.
-
- Eisenhauer EA, Ten Bokkel Huinink WW, Swenerton KD, Gianni L, Myles J, Van der Burg ME, et al. European-Canadian randomized trial of paclitaxel in relapsed ovarian cancer: high-dose versus low-dose and long versus short infusion. Journal of Clinical Oncology 1994;12(12):2654-66. [DOI: 10.1200/jco.1994.12.12.2654] - DOI - PubMed
-
- Ten Bokkel Huinink WW, Eisenhauer E, Swenerton K, Canadian-European Taxol Cooperative Trial group. Preliminary evaluation of a multicenter, randomized comparative study of TAXOL (paclitaxel) dose and infusion length in platinum-treated ovarian cancer. Cancer Treatment Reviews 1993;19 Suppl C(Suppl 3):79-86. - PubMed
Omura 2003 {published data only}
-
- Omura GA, Brady MF, Delmore JE, Long HJ, Look KY, Averette E, et al. A randomized trial of paclitaxel (T) at 2 dose levels and filgrastim (G; G-CSF) at 2 doses in platinum (P) pretreated epithelial ovarian cancer (OVCA): a Gynecologic Oncology Group, SWOG, NCCTG and ECOG study. American Society of Clinical Oncology 1996;15:280, Abstract 755. - PubMed
-
- Omura GA, Brady MF, Look KY, Averette HE, Delmore JE, Long HJ, et al. Phase III trial of paclitaxel at two dose levels, the higher dose accompanied by filgrastim at two dose levels in platinum-pretreated epithelial ovarian cancer: an intergroup study. Journal of Clinical Oncology 2003;21(15):2843-8. [DOI: 10.1200/JCO.2003.10.082] - DOI - PubMed
Osman 2016 {published data only}
Rosenberg 2002 {published data only}
-
- Andersson H, Boman K, Ridderheim P, Rosenberg P, Sorbe U, Puistola U. An updated analysis of a randomised study of single agent paclitaxel (P) given weekly versus every 3 weeks to patients (Pts) with ovarian cancer (OV) treated with prior platinum therapy. In: American Society of Clinical Oncology; 2000. 2000.
-
- Rosenberg P, Andersson H, Boman K, Ridderheim M, Sorbe B, Puistola U, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncologica (Stockholm, Sweden) 2002;41(5 CNO):418-24. - PubMed
References to studies excluded from this review
Cantu 2002 {published data only}
-
- Cantu MG, Buda A, Parma G, Rossi R, Floriani I, Bonazzi C, et al. Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. Journal of Clinical Oncology 2002;20(5):1232-7. - PubMed
Conte 2007 {published data only}
-
- Conte PF, Favalli G, Gadducci A, Katsaros D, Benedetti Panici PL, Carpi A, et al. Final results of After-6 protocol 1: a phase III trial of observation versus 6 courses of paclitaxel (Pac) in advanced ovarian cancer patients in complete response (CR) after platinum-paclitaxel chemotherapy (CT). Journal of Clinical Oncology 2007;25(18 Suppl):5505. - PubMed
Du Bois 1997 {published data only}
-
- Du Bois A, Luck HJ, Buser K, Meerpohl HG, Sessa C, Klaassen U, et al. Extended phase II study of paclitaxel as a 3-h infusion in patients with ovarian cancer previously treated with platinum. European Journal of Cancer 1997;33(3):379-84. - PubMed
Favalli 2002 {published data only}
-
- Favalli G, Conte PF, Katzaros D, Zola P, Nanni O, Gadducci Angiolo, et al. Randomized phase III trial of observation versus 6 courses of single agent paclitaxel in patients with advanced ovarian cancer who attained a complete response to platinum-paclitaxel based chemotherapy. An Italian study (AFTER-6 protocol 1). Journal of Clinical Oncology 2002;27(28):4642-8. - PubMed
Huizing 1993 {published data only}
-
- Huizing MT, Keung AC, Rosing H, Van der Kuij V, Ten Bokkel Huinink WW, Mandjes IM, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. Journal of Clinical Oncology 1993;11:2127-35. [DOI: 10.1200/jco.1993.11.11.2127] - DOI - PubMed
Ledermann 2003 {published data only}
-
- Ledermann JA. Randomized trial of paclitaxel in combination with platinum chemotherapy versus platinum/based chemotherapy in the treatment of relapsed ovarian cancer (ICON4/OVAR 2.2). American Society of Clinical Oncology 2003:446 Abstract no. 1794.
Markman 2002 {published data only}
-
- Markman M, Hall J, Spitz D, Weiner S, Carson L, Van Le L, et al. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. Journal of Clinical Oncology 2002;20(9):2365-9. - PubMed
Markman 2003 {published data only}
-
- Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. Journal of Clinical Oncology 2003;21(13):2460-5. - PubMed
Mayerhofer 1997 {published data only}
-
- Mayerhofer K, Kucera E, Zeisler H, Speiser P, Reinthaller A, Sevelda P. Taxol as second-line treatment in patients with advanced ovarian cancer after platinum-based first-line chemotherapy. Gynecologic Oncology 1997;64(1):109-13. - PubMed
Nardi 1997 {published data only}
-
- Nardi M, Aloe A, De Marco S, Cognetti F, Iacovelli A, Atlante G, et al. Paclitaxel as salvage therapy in advanced pretreated ovarian cancer: a phase II study. American Journal of Clinical Oncology 1997;20(3):230-2. - PubMed
Pecorelli 2009 {published data only}
-
- Pecorelli S, Favalli G, Gadducci A, Katsaros D, Panici PB, Carpi A, et al. Phase III trial of observation versus six courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after six courses of paclitaxel/platinum-based chemotherapy: final results of the After-6 protocol. Journal of Clinical Oncology 2009;27(28):4642-6. - PubMed
Piccart 2000 {published data only}
-
- Piccart MJ, Green JA, Lacave AJ, Reed N, Vergote I, Benedetti-Panici P, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology group. Journal of Clinical Oncology 2000;18(6):1193-202. - PubMed
Tinker 2007 {published data only}
-
- Tinker AV, Gebski V, Fitzharris B, Buck M, Stuart-Harris R, Beale P, et al. Phase II trial of weekly docetaxel for patients with relapsed ovarian cancer who have previously received paclitaxel-ANZGOG 02-01. Gynecologic Oncology 2007;104(3):647-53. - PubMed
Torri 2000 {published data only}
-
- Torri V, Floriani I, Tinazzi A, Conte PF, Ravaioli A, Cantu MG, et al. Randomized trial comparing paclitaxel + doxorubicin (AT) versus paclitaxel (T) as second line therapy for advanced ovarian cancer (AOC) patients in early progression after platinum based chemotherapy. American Society of Clinical Oncology (proceedings) 2000;19:381a, Abstract 1506.
Trope 1998 {published data only}
-
- Trope C, Hogberg T, Kaern J, Bertelsen K, Bjorkholm E, Boman K, et al. Long-term results from a phase II study of single agent paclitaxel (taxol) in previously platinum treated patients with advanced ovarian cancer: the Nordic experience. Annals of Oncology 1998;9(12):1301-7. - PubMed
Additional references
Alberts 2002
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Mitosis. In: Molecular Biology of the Cell. 4th edition. New York, US: Garland Science, 2002.
Andersson 1997
-
- Andersson H, Horvath G, Mellqvist L, Westberg R. Taxol given weekly in advanced previously treated ovarian carcinomas - a pilot study. International Journal of Gynecological Cancer 1997;7(4):262-6.
Bruzzone 1996
-
- Bruzzone M, Catsafados E, Miglietta L, Amoroso D, Pedulla F, Giannessi PG, et al. Salvage chemotherapy with paclitaxel in platinum-resistant advanced ovarian cancer patients. Oncology 1996;53(5):349-53. - PubMed
Cancer Research UK
-
- Cancer Research UK. Cancer incidence statistics. cancerresearchuk.org/health-professional/cancer-statistics/incidence (accessed 9 April 2021).
Cella 1993
-
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570-9. - PubMed
Chan 2016
Clamp 2019
-
- Clamp AR, James EC, McNeish IA, Dean A, Kim JW, O'Donnell DM, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet 2019;394(10214):2084-95. - PMC - PubMed
Covidence Systematic Review Software [Computer program]
-
- Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation, 2020.
Desai 2014
Donehower 1996
-
- Donehower RC. The clinical development of paclitaxel: a successful collaboration of academia, industry and the National Cancer Institute. Oncologist 1996;1(4):240-3. - PubMed
Dunder 2005
-
- Dunder I, Berker B, Atabekoglu C, Bilgin T. Preliminary experience with salvage weekly paclitaxel in women with advanced recurrent ovarian carcinoma. European Journal of Gynaecological Oncology 2005;26(1):79-82. - PubMed
EUROCARE 2003
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al, EUROCARE Working Group. EUROCARE-3: survival of cancer patients diagnosed 1990–94 – results and commentary. Annals of Oncology 2003;14(Suppl 5):v61-118. - PubMed
Ezcurdia 1997
-
- Ezcurdia L, Jovtis SL, Mickiewicz E, Temperley G, Rondinon M, Blajman C, et al, Argentine Multicenter Taxol group. Paclitaxel in platinum-resistant ovarian cancer patients.. Seminars in Oncology 1997;24(5 Suppl 15):S15. - PubMed
Fennelly 1997
-
- Fennelly D, Aghajanian C, Shapiro F, O'Flaherty C, McKenzie M, O'Connor C, et al. Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer. Journal of Clinical Oncology 1997;15(1):187-92. - PubMed
Ferlay 2021
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. International Journal of Cancer 2021;149(4):778-89. - PubMed
Friedlander 2011
-
- Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, et al. Clinical trials in recurrent ovarian cancer. International Journal of Gynecological Cancer 2011;21(4):771-5. - PubMed
Ghamande 2003
-
- Ghamande S, Lele S, Marchetti D, Baker T, Odunsi K. Weekly paclitaxel in patients with recurrent or persistent advanced ovarian cancer. International Journal of Gynecological Cancer 2003;13(2):142-7. - PubMed
Gianni 1995
-
- Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Locatelli A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. Journal of Clinical Oncology 1995;13(1):180-90. - PubMed
Gligorov 2004
-
- Gligorov J, Lotz JP. Preclinical pharmacology of the taxanes: implications of the differences. Oncologist 2004;9(S2):3-8. - PubMed
Gore 1995
Gore 1995a
GRADEPro GDT [Computer program]
-
- GRADEpro GDT. Version (accessed 9 April 2018). Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grant 2003
-
- Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). International Journal of Cancer 2003;104(1):121-9. - PubMed
Hashiguchi 2015
Hernadi 2001
-
- Hernadi Z, Huga S, Lukacsko L, Krasznai Z, Sapy T. Chemotherapy resistance of ovarian cancer patients after platinum-based therapy paclitaxel as a choice of treatment. [Hungarian]. Magyar Noorvosok Lapja 2001;64(3):249-54.
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook.
Hoekman 1995
-
- Hoekman K, Huijskes RV, Burger CW, Verheijen RH, Pinedo HM, Vermorken JB. [Favorable results of paclitaxel (taxol) in patients with ovary carcinoma pretreated with platinum] [Dutch]. Nederlands Tijdschrift voor Geneeskunde 1995;139(6):272-8. - PubMed
Hsu 2004
-
- Hsu Y, Sood AK, Sorosky JI. Docetaxel versus paclitaxel for adjuvant treatment of ovarian cancer: case-control analysis of toxicity. American Journal of Clinical Oncology 2004;27(1):14-8. - PubMed
Ishikawa 2001
-
- Ishikawa H, Nakanishi T, Nawa A, Suzuki Y, Kuzuya K. 3-hour infusion of single-agent paclitaxel for recurrent ovarian cancer. International Journal of Clinical Oncology 2001;6(3):128-31. - PubMed
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: a Cancer Journal for Clinicians 2008;58(2):71-96. - PubMed
Jemal 2011
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a Cancer Journal for Clinicians 2011;61:69-90. - PubMed
Jordan 2004
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews. Cancer 2004;4(4):253-65. - PubMed
Kaern 2002
-
- Kaern J, Baekelandt M, Tropé CG. A phase II study of weekly paclitaxel in platinum and paclitaxel-resistant ovarian cancer patients. European Journal of Gynaecological Oncology 2002;23(5):383-9. - PubMed
Katsumata 2013
-
- Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncology 2013;14(10):1020-6. - PubMed
Kita 2004
-
- Kita T, Kikuchi Y, Takano M, Suzuki M, Oowada M, Konno R, et al. The effect of single weekly paclitaxel in heavily pretreated patients with recurrent or persistent advanced ovarian cancer. Gynecologic Oncology 2004;92(3):813-8. - PubMed
Kohn 1994
-
- Kohn EC, Sarosy G, Bicher A, Link C, Christian M, Steinberg SM, et al. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. Journal of the National Cancer Institute 1994;86(1):18-24. - PubMed
Kristensen 2008
-
- Kristensen G, Kaern J, Baekelandt M, Skeie-Jensensen T, De Pont Christensen R, Avall-Lundqvist E, et al. Chemotherapy versus hormonal treatment in patients with platinum and taxane resistant ovarian cancer: a NSGO study. Journal of Clinical Oncology (ASCO Annual Meeting Proceedings) 2008;26(Suppl):Abstract.
Kudelka 1999
-
- Kudelka A, Verschraegen C, Shen Y, De Leon C, Edwards C, Freedman R, et al. Long-term results and pharmacokinetics of high-dose paclitaxel in patients with refractory epithelial ovarian carcinoma. International Journal of Gynaecological Cancer 1999;9(1):44-53. - PubMed
Langendam 2013
Le 2006
-
- Le T, Hopkins L, Baines KA, Rambout L, Al Hayki M, Kee Fung MF. Prospective evaluations of continuous weekly paclitaxel regimen in recurrent platinum-resistant epithelial ovarian cancer. Gynecologic Oncology 2006;102(1):49-53. - PubMed
Liberati 2009
Litière 2016
-
- Litière S, Collette S, De Vries EG, Seymour L, Bogaerts J. RECIST - learning from the past to build the future. Nature Reviews. Clinical Oncology 2017;14(3):187-92. - PubMed
Lortholary 2012
-
- Lortholary A, Largillier R, Weber B, Gladieff L, Alexandre J, Durando X, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: the CARTAXHY randomized phase II trial from Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens (GINECO). Annals of Oncology 2012;23(2):346-52. - PubMed
Markman 2006
-
- Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;101(3):436-40. - PubMed
McGuire 1989
-
- McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Annals of Internal.Medicine 1989;111(4):273-9. - PubMed
McGuire 1996
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine 1996;334(1):1-6. - PubMed
Meader 2014
Meinhold‐Heerlein 2016
-
- Meinhold-Heerlein I, Fotopoulou C, Harter P, Kurzeder C, Mustea A, Wimberger P, et al. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Archives of Gynecology and Obstetrics 2016;293(4):695-700. - PubMed
MSD Manual 2021
-
- Territo M. Neutropenia - Hematology and Oncology. MSD Manual Professional Version. Available from www.msdmanuals.com/en-gb/professional/hematology-and-oncology/leukopenia... 2021 (accessed prior to 12 June 2022).
NICE 2005
-
- NICE. Guidance on the use of paclitaxel in the treatment of ovarian cancer: Technology appraisal guidance [TA55] Published date: 22 January 2003 Last updated: 01 May 2005. www.nice.org.uk/guidance/ta55 (accessed prior to 12 June 2022).
NICE 2016
-
- NICE. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for treating recurrent ovarian cancer: Technology appraisal guidance [TA389]. www.nice.org.uk/guidance/ta389 (accessed prior to 12 June 2022).
NICE 2020
-
- NICE. Olaparib for maintenance treatment of relapsed platinum-sensitive ovarian, fallopian tube or peritoneal cancer. Technology appraisal guidance [TA620]. www.nice.org.uk/guidance/ta620 (accessed prior to 12 June 2022).
Noda 1994
-
- Noda K, Ikeda M, Kudo R, Nishiya I, Yajima A, Tanaka K, et al, BMS-181339 ovarian cancer study group. [A phase II study of BMS-181339 in patients with ovarian cancer] [Japanese]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1994;21(14):2461-9. - PubMed
Noda 1996
-
- Noda K, Ikeda M, Kudo R, Nishiya I, Yajima A, Tanaka K, et al. [Phase II study of paclitaxel (BMS-181339) in patients with ovarian cancer by 3-hour intravenous infusion] [Japanese]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1996;23(3):317-25. - PubMed
Oliverio 1999
-
- Oliverio G, Canuti D, Tononi A, Scarpellini M, Panzini I, Galli I, et al. Paclitaxel efficacy and tolerability in second-line treatment of refractory and relapsed ovarian cancer patients. Journal of Chemotherapy 1999;11(4):301-5. - PubMed
Ozols 2006
-
- Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Seminars in Oncology 2006;33(2 Suppl 6):S3-11. - PubMed
Park 2003
-
- Park JO, Lee SI, Song SY, Kim K, Kim WS, Jung CW, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Japanese Journal of Clinical Oncology 2003;33(10):533-7. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. - PubMed
Pectasides 1998
-
- Pectasides D, Papadopoulou M, Varthalitis J, Mylonakis A, Kostopoulou M, Dimitriadis M, et al. Paclitaxel in cisplatin or carboplatin-pretreated ovarian cancer. Phase II study. Oncology 1998;55(3):228-34. - PubMed
Phillips 1995
-
- Phillips KA, Friedlander M, Olver I, Evans B, Smith J, Fitzharris B, et al. Australasian multicentre phase II study of paclitaxel (taxol) in relapsed ovarian cancer. Australian and New Zealand Journal of Medicine 1995;25(4):337-43. - PubMed
Pignata 2015
-
- Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncology 2015;16(5):561-8. - PubMed
Planner 1996
-
- Planner RS, Allen DG, Brand AH, Grant PT, Toner GC, Sykes PH. Paclitaxel (taxol) as salvage therapy for relapsed ovarian cancer. Australian & New Zealand Journal of Obstetrics & Gynaecology 1996;36(2):168-70. - PubMed
Review Manager 2022 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2022.
Sarosy 1992
-
- Sarosy G, Kohn E, Stone DA, Rothenberg M, Jacob J, Adamo DO, et al. Phase I study of taxol and granulocyte colony-stimulating factor in patients with refractory ovarian cancer. Journal of Clinical Oncology 1992;10(7):1165-70. - PubMed
Schünemann 2020
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/current/chapter-14. [www.cochrane-handbook.org]
Spitzer 1981
-
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. Journal of Chronic Diseases 1981;34(12):585-97. - PubMed
Stordal 2007a
-
- Stordal B, Pavlakis N, Davey R. A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship. Cancer Treatment Reviews 2007;33(8):688-703. - PubMed
Stordal 2007b
-
- Stordal B, Pavlakis N, Davey R. Oxaliplatin for the treatment of cisplatin-resistant cancer: a systematic review. Cancer Treatment Reviews 2007;33(4):347-57. - PubMed
Stordal 2007c
-
- Stordal B, Davey M. Understanding cisplatin resistance using cellular models. International Union of Biochemistry & Molecular Biology Life 2007;59(11):696-9. - PubMed
Takano 2002
-
- Takano M, Kikuchi Y, Kita T, Suzuki M, Ohwada M, Yamamoto T, et al. Phase I and pharmacological study of single paclitaxel administered weekly for heavily pre-treated patients with epithelial ovarian cancer. Anticancer Research 2002;22(3):1833-8. - PubMed
Ten Bokkel Huinink 1997
-
- Ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. Journal of Clinical Oncology 1997;15(6):2183-93. - PubMed
Thigpen 1994
-
- Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. Journal of Clinical Oncology 1994;12(9):1748-53. - PubMed
Tierney 2007
Trimble 1993
-
- Trimble EL, Adams JD, Vena D, Hawkins MJ, Friedman MA, Fisherman JS, et al. Paclitaxel for platinum-refractory ovarian cancer: results from the first 1,000 patients registered to National Cancer Institute Treatment Referral Center 9103. Journal of Clinical Oncology 1993;11(12):2405-10. - PubMed
Trotti 2003
-
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology 2003;13(3):176-81. - PubMed
Vacca 2002
-
- Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R, et al. Docetaxel versus paclitaxel for antiangiogenesis. Journal of Hematotherapy & Stem Cell Research 2002;11(1):103-18. - PubMed
Vang 2013
-
- Vang R, Shih Le-M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology 2013;62(1):44-58. - PubMed
Vasey 2004
-
- Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. Journal of the National Cancer Institute 2004;96(22):1682-91. - PubMed
Vermorken 2001
-
- Vermorken JB. The integration of paclitaxel and new platinum compounds in the treatment of advanced ovarian cancer. International Journal of Gynecological Cancer 2001;11 Suppl 1:21-30. - PubMed
Verweij 1994
-
- Verweij J, Clavel M, Chevalier B. Paclitaxel (taxol) and docetaxel (taxotere): not simply two of a kind. Annals of Oncology 1994;5(6):495-505. - PubMed
Wilailak 2000
-
- Wilailak S, Tresukosol D, Linasmita V, Termrungruanglert W. Phase II study of high-dose paclitaxel in platinum-refractory epithelial ovarian cancer. European Journal of Gynaecological Oncology 2000;21(6):610-2. - PubMed
Zeppernick 2014
-
- Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Archives of Gynecology and Obstetrics 2014;290(5):839-42. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

