Comparison of animal and human blood for in vitro dynamic thrombogenicity testing of biomaterials
- PMID: 35866431
- PMCID: PMC9669094
- DOI: 10.1111/aor.14366
Comparison of animal and human blood for in vitro dynamic thrombogenicity testing of biomaterials
Abstract
Background: To determine suitable alternatives to human blood for in vitro dynamic thrombogenicity testing of biomaterials, four different animal blood sources (ovine, bovine, and porcine blood from live donors, and abattoir porcine blood) were compared to fresh human blood.
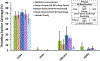
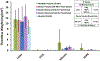
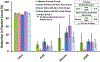
Methods: To account for blood coagulability differences between individual donors and species, each blood pool was heparinized to a donor-specific concentration immediately before testing in a dynamic flow loop system. The target heparin level was established using a static thrombosis pre-test. For dynamic testing, whole blood was recirculated at room temperature for 1 h at 200 ml/min through a flow loop containing a single test material. Four materials with varying thrombotic potentials were investigated: latex (positive control), polytetrafluoroethylene (PTFE) (negative control), silicone (intermediate thrombotic potential), and high-density polyethylene (HDPE) (historically thromboresistant). Thrombus weight and surface area coverage on the test materials were quantified, along with platelet count reduction in the blood.
Results: While donor-specific heparin levels varied substantially from 0.6 U/ml to 7.0 U/ml among the different blood sources, each source was able to differentiate between the thrombogenic latex and the thromboresistant PTFE and HDPE materials (p < 0.05). However, only donor ovine and bovine blood were sensitive enough to differentiate an increased response for the intermediate thrombotic silicone material compared to PTFE and HDPE.
Conclusions: These results demonstrated that multiple animal blood sources (particularly donor ovine and bovine blood) may be suitable alternatives to fresh human blood for dynamic thrombogenicity testing when appropriate control materials and donor-specific anticoagulation levels are used.
Keywords: animal blood sources; blood flow loop; human blood; in vitro testing; platelets; thrombogenicity; thrombosis.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA.
Figures



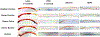
References
-
- Wiper A, Hashmi I, Srivastava V, Shaktawat S, Sogliani F, Tang G, et al. Guide wire thrombus formation during trans-femoral TAVI. Cardiovasc Revasc Med 2014;15(6–7):360–1. - PubMed
-
- Chin T, Priyesh P, Islam AM. A novel approach to extraction of a large thrombus on the intraventricular guide-wire during transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2017;89(3):495–8. - PubMed
-
- Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125(24):3038–47. - PubMed
-
- Li S, Henry JJ. Nonthrombogenic approaches to cardiovascular bioengineering. Annu Rev Biomed Eng 2011;13:451–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

