PTP1B inhibitors protect against acute lung injury and regulate CXCR4 signaling in neutrophils
- PMID: 35866483
- PMCID: PMC9431713
- DOI: 10.1172/jci.insight.158199
PTP1B inhibitors protect against acute lung injury and regulate CXCR4 signaling in neutrophils
Abstract
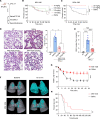
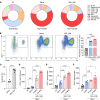
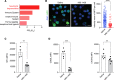
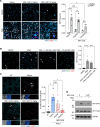
Acute lung injury (ALI) can cause acute respiratory distress syndrome (ARDS), a lethal condition with limited treatment options and currently a common global cause of death due to COVID-19. ARDS secondary to transfusion-related ALI (TRALI) has been recapitulated preclinically by anti-MHC-I antibody administration to LPS-primed mice. In this model, we demonstrate that inhibitors of PTP1B, a protein tyrosine phosphatase that regulates signaling pathways of fundamental importance to homeostasis and inflammation, prevented lung injury and increased survival. Treatment with PTP1B inhibitors attenuated the aberrant neutrophil function that drives ALI and was associated with release of myeloperoxidase, suppression of neutrophil extracellular trap (NET) formation, and inhibition of neutrophil migration. Mechanistically, reduced signaling through the CXCR4 chemokine receptor, particularly to the activation of PI3Kγ/AKT/mTOR, was essential for these effects, linking PTP1B inhibition to promoting an aged-neutrophil phenotype. Considering that dysregulated activation of neutrophils has been implicated in sepsis and causes collateral tissue damage, we demonstrate that PTP1B inhibitors improved survival and ameliorated lung injury in an LPS-induced sepsis model and improved survival in the cecal ligation and puncture-induced (CLP-induced) sepsis model. Our data highlight the potential for PTP1B inhibition to prevent ALI and ARDS from multiple etiologies.
Keywords: Cell Biology; Neutrophils.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

