Histological evidence supporting the durability of successful radiofrequency renal denervation in a normotensive porcine model
- PMID: 35866489
- PMCID: PMC9451943
- DOI: 10.1097/HJH.0000000000003236
Histological evidence supporting the durability of successful radiofrequency renal denervation in a normotensive porcine model
Abstract
Background: Sustained blood pressure reductions after radiofrequency (RF) renal denervation (RDN) have been reported to 3 years in patients with uncontrolled hypertension. However, mechanistic data to support procedural durability are lacking. We aimed to quantify the long-term nerve anatomic and functional effects of RF RDN in a preclinical model.
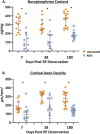
Methods: Bilateral RF RDN was performed in 20 normotensive swine. Renal tissue samples were obtained in the RDN-treated groups at 7 ( n = 6), 28 ( n = 6), and 180 days ( n = 8) postprocedure for quantification of cortical norepinephrine (NE) levels and renal cortical axon density. Tissue fibrosis, necrosis and downstream nerve fiber atrophy (axonal loss) were also scored for each sample. Three additional untreated groups ( n = 6, n = 6 and n = 8, respectively) served as control.
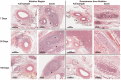
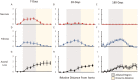
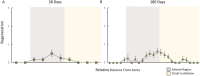
Results: Pathologic nerve changes were characterized by necrosis in the ablated region at 7 days that partially resolved by 28 days and fully resolved at 180 days. Axonal loss was apparent within and downstream to the ablation regions and was evident at 7, 28 and 180 days in the main vessel and branch vessels. Consequently, renal cortical axon density and corresponding cortical NE levels were significantly reduced at 7 days in the RDN vs. control group and remained suppressed at 180 days.
Conclusions: Reductions in renal NE, cortical axon density and downstream axonal loss caused by axonal destruction persisted through 180 days post-RDN in a normotensive swine model. These results suggest functional nerve regrowth after RF RDN is unlikely and support published clinical evidence that the procedure results in durable blood pressure reduction.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Funding: This work was funded by Medtronic.
Disclosures: A.S.P.S. receives consulting fees/honoraria from Medtronic, Philips and Recor Medical.
S.T. is an employee of Medtronic.
M.S. is supported by an NHMRC Senior Research Fellowship; and has received consulting fees and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim.
D.P.L. reports grants from and serves on the advisory board for Medtronic.
A.V.F. reports consulting honoraria from: Amgen; Abbott Vascular; Biosensors; Boston Scientific; Celonova; Cook Medical; CSI; Lutonix Bard; Medtronic, Terumo Corporation and Institutional grant/research support from R01 HL141425 Leducq Foundation Grant; 480 Biomedical; 4C Medical; 4Tech; Abbott; Accumedical; Amgen; Biosensors; Boston Scientific; Cardiac Implants; Celonova; Claret; Concept Medical; Cook; CSI; DuNing; Edwards; Emboline; Endotronix; Envision Scientific; Lutonix/Bard; Gateway; Lifetech; Limflo; MedAlliance; Medtronic; Mercator; Merill; Microport; Microvention; Mitraalign; Mitra assist; NAMSA; Nanova; Neovasc; NIPRO; Novogate; Occulotech; Orbus Neich; Phenox; Profusa; Protembis; Qool; Recor; Senseonics; Shockwave; Sinomed; Spectranetics; Surmodics; Symic; Vesper; W.L. Gore; Xeltis.
J.T. is an employee of Medtronic.
D.A.H. is an employee of Medtronic.
F.M. is supported by Deutsche Gesellschaft für Kardiologie; and has received scientific support and speaker honoraria from Bayer, Boehringer Ingelheim, Medtronic, and ReCor Medical.
D.E.K. reports institutional research/grant support from Medtronic CardioVascular and Ablative Solutions and personal consulting honoraria from Medtronic CardioVascular.
Figures



References
-
- Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; 391:2346–2355. - PubMed
-
- Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020; 395:1444–1451. - PubMed
-
- Azizi M, Pereira H, Hamdidouche I, Gosse P, Monge M, Bobrie G, et al. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the Renal Denervation for Hypertension (DENERHTN) Trial. Circulation 2016; 134:847–857. - PubMed
-
- Azizi M, Sanghvi K, Saxena M, Gosse P, Reilly JP, Levy T, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021; 397:2476–2486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

