Multi-population generalizability of a deep learning-based chest radiograph severity score for COVID-19
- PMID: 35866818
- PMCID: PMC9302282
- DOI: 10.1097/MD.0000000000029587
Multi-population generalizability of a deep learning-based chest radiograph severity score for COVID-19
Abstract
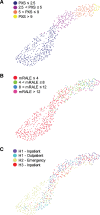
To tune and test the generalizability of a deep learning-based model for assessment of COVID-19 lung disease severity on chest radiographs (CXRs) from different patient populations. A published convolutional Siamese neural network-based model previously trained on hospitalized patients with COVID-19 was tuned using 250 outpatient CXRs. This model produces a quantitative measure of COVID-19 lung disease severity (pulmonary x-ray severity (PXS) score). The model was evaluated on CXRs from 4 test sets, including 3 from the United States (patients hospitalized at an academic medical center (N = 154), patients hospitalized at a community hospital (N = 113), and outpatients (N = 108)) and 1 from Brazil (patients at an academic medical center emergency department (N = 303)). Radiologists from both countries independently assigned reference standard CXR severity scores, which were correlated with the PXS scores as a measure of model performance (Pearson R). The Uniform Manifold Approximation and Projection (UMAP) technique was used to visualize the neural network results. Tuning the deep learning model with outpatient data showed high model performance in 2 United States hospitalized patient datasets (R = 0.88 and R = 0.90, compared to baseline R = 0.86). Model performance was similar, though slightly lower, when tested on the United States outpatient and Brazil emergency department datasets (R = 0.86 and R = 0.85, respectively). UMAP showed that the model learned disease severity information that generalized across test sets. A deep learning model that extracts a COVID-19 severity score on CXRs showed generalizable performance across multiple populations from 2 continents, including outpatients and hospitalized patients.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Conflicts of interest and sources of funding: This study was supported by sundry funds to J.K. This research was carried out in whole or in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health. GPU computing resources were provided by the MGH and BWH Center for Clinical Data Science. M.D.L., B.C.B., I.D., and J.K. report collaborating with Bayer Radiology on addressing regulatory requirements for potential clinical application of this technology (no funding provided for the work in this manuscript). M.D.L. reports funding from an RSNA R&E Fund Research Resident/Fellow Grant, outside of the submitted work. BPL is a textbook associate editor and author for Elsevier, Inc. and receives royalties. F.C.K. reports consulting for MD.ai. J.K. reports grants from GE Healthcare, nonfinancial support from AWS, and grants from Genentech Foundation, outside the submitted work. For the remaining authors none were declared.
Figures


Update of
-
Improvement and Multi-Population Generalizability of a Deep Learning-Based Chest Radiograph Severity Score for COVID-19.medRxiv [Preprint]. 2020 Sep 18:2020.09.15.20195453. doi: 10.1101/2020.09.15.20195453. medRxiv. 2020. Update in: Medicine (Baltimore). 2022 Jul 22;101(29):e29587. doi: 10.1097/MD.0000000000029587. PMID: 32995811 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

