Health economic evaluation of an mRNA high-risk human papillomavirus (HR-HPV) assay versus a DNA HR-HPV assay for the proposed French cervical screening programme
- PMID: 35866838
- PMCID: PMC9302372
- DOI: 10.1097/MD.0000000000029530
Health economic evaluation of an mRNA high-risk human papillomavirus (HR-HPV) assay versus a DNA HR-HPV assay for the proposed French cervical screening programme
Abstract
Objective: Population screening programmes must make good use of resources for the health system and users. To evaluate impacts of the type of diagnostic test in the new French cervical screening programme, an messenger ribonucleic acid (mRNA) high-risk human papillomavirus assay was compared to a deoxyribonucleic acid (DNA) high-risk human papillomavirus assay for a hypothetical cohort of women aged 25 to 65 years.
Perspective: This evaluation takes the perspective of the French healthcare system.
Setting: France.
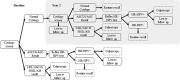
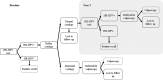
Methods: A decision tree model reflecting the French cervical screening algorithms was parametrised using French cost and population data and the Danish Horizon study. The outcomes were total costs, and number of colposcopies, HPV tests and cytology tests for the cohort. One-way and probabilistic sensitivity analyses and scenarios analyses were conducted to test the robustness of results to parameter and structural uncertainty.
Results: Adopting an mRNA versus DNA assay as part of national cervical screening in France is estimated to save €6.5 million (95% credibility intervals €-1.3 - €13.5 million) and prevent 47,795 (95% credibility intervals 35,309 - 60,139) unnecessary colposcopies, 38,666 unnecessary HPV tests and 121,670 cytology tests over two years for a cohort of 2,168,806 million women aged 25 to 65 years. Sensitivity analyses indicated robust results across a range of inputs.
Conclusion: The choice of high-risk human papillomavirus assay makes a significant difference to resource use and costs and is important to consider when implementing cervical screening in France. Using an mRNA versus DNA assay can result in cost savings and reductions in unnecessary testing and procedures, which in turn benefits women and the health care system.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


Similar articles
-
Use of the Aptima mRNA high-risk human papillomavirus (HR-HPV) assay compared to a DNA HR-HPV assay in the English cervical screening programme: a decision tree model based economic evaluation.BMJ Open. 2020 Mar 8;10(3):e031303. doi: 10.1136/bmjopen-2019-031303. BMJ Open. 2020. PMID: 32152154 Free PMC article.
-
[Health technology assessment report: HPV DNA based primary screening for cervical cancer precursors].Epidemiol Prev. 2012 May-Aug;36(3-4 Suppl 1):e1-72. Epidemiol Prev. 2012. PMID: 22828243 Italian.
-
The Clinical and Economic Benefits of Co-Testing Versus Primary HPV Testing for Cervical Cancer Screening: A Modeling Analysis.J Womens Health (Larchmt). 2016 Jun;25(6):606-16. doi: 10.1089/jwh.2015.5708. Epub 2016 Mar 29. J Womens Health (Larchmt). 2016. PMID: 27023044 Free PMC article.
-
Accuracy and effectiveness of HPV mRNA testing in cervical cancer screening: a systematic review and meta-analysis.Lancet Oncol. 2022 Jul;23(7):950-960. doi: 10.1016/S1470-2045(22)00294-7. Epub 2022 Jun 13. Lancet Oncol. 2022. PMID: 35709810
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
Cited by
-
Agreement between Self- and Physician‑Sampling for Detection of High‑Risk Human Papillomavirus Infections in Women Attending Cervical Screening at National Cancer Institute, Thailand.Asian Pac J Cancer Prev. 2023 Aug 1;24(8):2615-2619. doi: 10.31557/APJCP.2023.24.8.2615. Asian Pac J Cancer Prev. 2023. PMID: 37642046 Free PMC article.
-
An economic evaluation of two cervical screening algorithms in Belgium: HR-HPV primary compared to HR-HPV and liquid-based cytology co-testing.Eur J Cancer Prev. 2024 May 1;33(3):262-270. doi: 10.1097/CEJ.0000000000000856. Epub 2023 Nov 23. Eur J Cancer Prev. 2024. PMID: 37933867 Free PMC article.
-
RNA extended interventional nucleic acid longitudinal study: Clinical performance of Aptima messenger RNA HPV testing in cervical cancer screening with a 9-year follow-up.Cancer Cytopathol. 2024 Dec;132(12):757-767. doi: 10.1002/cncy.22895. Epub 2024 Aug 19. Cancer Cytopathol. 2024. PMID: 39158405 Free PMC article.
-
Performance of the Human Papillomavirus E6/E7 mRNA Assay in the Primary Screening of Cervical Cancer: Opportunistic Screening in Fujian, China.Int J Womens Health. 2022 Oct 25;14:1519-1530. doi: 10.2147/IJWH.S383431. eCollection 2022. Int J Womens Health. 2022. PMID: 36317009 Free PMC article.
-
Molecular detection of hrHPV-induced high-grade squamous intraepithelial lesions of the cervix through a targeted RNA next generation sequencing assay.Mol Med. 2025 May 30;31(1):215. doi: 10.1186/s10020-025-01238-x. Mol Med. 2025. PMID: 40448246 Free PMC article.
References
-
- Bruni L, Albero G, Serrano B, et al. . Human papillomavirus and related diseases in France. Summary Report ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre) 2019.
-
- Monsonego J, Bosch FX, Coursaget P, et al. . Cervical cancer control, priorities and new directions. Int J Cancer 2004;108:329–33. - PubMed
-
- Iftner T, Becker S, Neis K-J, et al. . Head-to-head comparison of the RNA-based aptima human papillomavirus (HPV) assay and the DNA-based hybrid capture 2 HPV test in a routine screening population of women aged 30 to 60 years in Germany. Tang Y-W, ed. J Clin Microbiol 2015;53:2509–16. - PMC - PubMed

