Astragalus polysaccharide mitigates transport stress-induced hepatic metabolic stress via improving hepatic glucolipid metabolism in chicks
- PMID: 35866893
- PMCID: PMC9492282
- DOI: 10.1093/jas/skac244
Astragalus polysaccharide mitigates transport stress-induced hepatic metabolic stress via improving hepatic glucolipid metabolism in chicks
Abstract
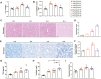
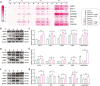
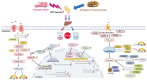
In the modern poultry industry, newly hatched chicks are unavoidably transported from the hatching to the rearing foster. Stress caused by multiple physical and psychological stressors during transportation is particularly harmful to the liver. Astragalus polysaccharide (APS) possesses multiple benefits against hepatic metabolic disorders. Given that transport stress could disturb hepatic glucolipid metabolism and the role of APS in metabolic regulation, we speculated that APS could antagonize transport stress-induced disorder of hepatic glucolipid metabolism. Firstly, newly hatched chicks were transported for 0, 2, 4, and 8 h, respectively. Subsequently, to further investigate the effects of APS on transport stress-induced hepatic glucolipid metabolism disturbance, chicks were pretreated with water or APS and then subjected to transport treatment. Our study suggested that APS could relieve transport stress-induced lipid deposition in liver. Meanwhile, transport stress also induced disturbances in glucose metabolism, reflected by augmented mRNA expression of key molecules in gluconeogenesis and glycogenolysis. Surprisingly, APS could simultaneously alleviate these alterations via peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α)/Sirtuin 1 (SIRT1)/AMP-activated protein kinase (AMPK) pathway. Moreover, APS treatment regulated the level of peroxisome proliferator-activated receptor alpha (PPARα) and peroxisome proliferator-activated receptor gamma (PPARγ), thereby alleviating transport stress-induced alterations of VLDL synthesis, cholesterol metabolism, lipid oxidation, synthesis, and transport-related molecules. These findings indicated that APS could prevent the potential against transport stress-induced hepatic glucolipid metabolism disorders via PGC-1α/SIRT1/AMPK/PPARα/PPARγ signaling system.
Keywords: Astragalus polysaccharide; glucolipid metabolism; hepatic steatosis; newly hatched chicks; transport stress.
Plain language summary
In the modern poultry industry, newly hatched chicks are unavoidably transported from the hatching to the rearing foster. During transportation, chicks are frequently subjected to various physical and psychological stressors, which can lead to alterations in blood composition, hormones, metabolites, enzymes, and behavior. These alterations adversely affect animal health and welfare. Stress caused by transportation is especially harmful to liver, which can cause significant effects on liver function, and disturb hepatic lipid metabolism and glucose metabolic. The current study demonstrated that Astragalus polysaccharide (APS) possesses multiple benefits against hepatic metabolic disorders. Administration of APS to chicks before transport could prevent transport-induced stress and hepatic glucolipid metabolism disorders.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1α/PPARα-FGF21 signaling pathway in male Sprague Dawley rats undergoing catch-up growth.Mol Med Rep. 2015 Nov;12(5):6451-60. doi: 10.3892/mmr.2015.4245. Epub 2015 Aug 25. Mol Med Rep. 2015. PMID: 26323321 Free PMC article.
-
Astragalus polysaccharide alleviates transport stress-induced heart injury in newly hatched chicks via ERS-UPR-autophagy dependent pathway.Poult Sci. 2022 Sep;101(9):102030. doi: 10.1016/j.psj.2022.102030. Epub 2022 Jun 23. Poult Sci. 2022. PMID: 35905545 Free PMC article.
-
LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway.World J Gastroenterol. 2019 Dec 7;25(45):6607-6618. doi: 10.3748/wjg.v25.i45.6607. World J Gastroenterol. 2019. PMID: 31832001 Free PMC article.
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
-
Peroxisome proliferator activated receptor α ligands as anticancer drugs targeting mitochondrial metabolism.Curr Pharm Biotechnol. 2013;14(3):342-56. doi: 10.2174/1389201011314030009. Curr Pharm Biotechnol. 2013. PMID: 21133850 Free PMC article. Review.
Cited by
-
Effect of compound polysaccharide on immunity, antioxidant capacity, gut microbiota, and serum metabolome in kittens.Front Microbiol. 2025 Mar 5;16:1500961. doi: 10.3389/fmicb.2025.1500961. eCollection 2025. Front Microbiol. 2025. PMID: 40109962 Free PMC article.
-
Essential oils improve nursery pigs' performance and appetite via modulation of intestinal health and microbiota.Anim Nutr. 2023 Dec 21;16:174-188. doi: 10.1016/j.aninu.2023.10.007. eCollection 2024 Mar. Anim Nutr. 2023. PMID: 38357573 Free PMC article.
-
Effects of dietary supplementation with a carvacrol-cinnamaldehyde-thymol blend on growth performance and intestinal health of nursery pigs.Porcine Health Manag. 2023 May 23;9(1):24. doi: 10.1186/s40813-023-00317-x. Porcine Health Manag. 2023. PMID: 37221604 Free PMC article.
-
Bioactive components and clinical potential of Astragalus species.Front Pharmacol. 2025 May 16;16:1585697. doi: 10.3389/fphar.2025.1585697. eCollection 2025. Front Pharmacol. 2025. PMID: 40453655 Free PMC article. Review.
References
-
- Anagnostopoulos, G., Motiño O., Li S., Carbonnier V., Chen H., Sica V., Durand S., Bourgin M., Aprahamian F., Nirmalathasan N., . et al. 2022. An obesogenic feedforward loop involving PPARγ, acyl-CoA binding protein and GABA(A) receptor. Cell Death Dis. 13:356. doi:10.1038/s41419-022-04834-5. - DOI - PMC - PubMed
-
- Burgess, S. C., Hausler N., Merritt M., Jeffrey F. M., Storey C., Milde A., Koshy S., Lindner J., Magnuson M. A., Malloy C. R., . et al. 2004. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279:48941–48949. doi:10.1074/jbc.M407120200. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

