Treatment intensification for metastatic prostate cancer: New treatment landscapes in androgen deprivation-based therapy
- PMID: 35866986
- PMCID: PMC9395319
- DOI: 10.1002/cac2.12340
Treatment intensification for metastatic prostate cancer: New treatment landscapes in androgen deprivation-based therapy
Conflict of interest statement
None
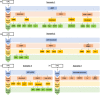
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

