Post-COVID-19 Conditions Among Children 90 Days After SARS-CoV-2 Infection
- PMID: 35867061
- PMCID: PMC9308058
- DOI: 10.1001/jamanetworkopen.2022.23253
Post-COVID-19 Conditions Among Children 90 Days After SARS-CoV-2 Infection
Erratum in
-
Error in Byline.JAMA Netw Open. 2022 Aug 1;5(8):e2231131. doi: 10.1001/jamanetworkopen.2022.31131. JAMA Netw Open. 2022. PMID: 35980643 Free PMC article. No abstract available.
Abstract
Importance: Little is known about the risk factors for, and the risk of, developing post-COVID-19 conditions (PCCs) among children.
Objectives: To estimate the proportion of SARS-CoV-2-positive children with PCCs 90 days after a positive test result, to compare this proportion with SARS-CoV-2-negative children, and to assess factors associated with PCCs.
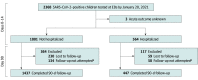
Design, setting, and participants: This prospective cohort study, conducted in 36 emergency departments (EDs) in 8 countries between March 7, 2020, and January 20, 2021, included 1884 SARS-CoV-2-positive children who completed 90-day follow-up; 1686 of these children were frequency matched by hospitalization status, country, and recruitment date with 1701 SARS-CoV-2-negative controls.
Exposure: SARS-CoV-2 detected via nucleic acid testing.
Main outcomes and measures: Post-COVID-19 conditions, defined as any persistent, new, or recurrent health problems reported in the 90-day follow-up survey.
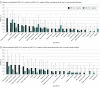
Results: Of 8642 enrolled children, 2368 (27.4%) were SARS-CoV-2 positive, among whom 2365 (99.9%) had index ED visit disposition data available; among the 1884 children (79.7%) who completed follow-up, the median age was 3 years (IQR, 0-10 years) and 994 (52.8%) were boys. A total of 110 SARS-CoV-2-positive children (5.8%; 95% CI, 4.8%-7.0%) reported PCCs, including 44 of 447 children (9.8%; 95% CI, 7.4%-13.0%) hospitalized during the acute illness and 66 of 1437 children (4.6%; 95% CI, 3.6%-5.8%) not hospitalized during the acute illness (difference, 5.3%; 95% CI, 2.5%-8.5%). Among SARS-CoV-2-positive children, the most common symptom was fatigue or weakness (21 [1.1%]). Characteristics associated with reporting at least 1 PCC at 90 days included being hospitalized 48 hours or more compared with no hospitalization (adjusted odds ratio [aOR], 2.67 [95% CI, 1.63-4.38]); having 4 or more symptoms reported at the index ED visit compared with 1 to 3 symptoms (4-6 symptoms: aOR, 2.35 [95% CI, 1.28-4.31]; ≥7 symptoms: aOR, 4.59 [95% CI, 2.50-8.44]); and being 14 years of age or older compared with younger than 1 year (aOR, 2.67 [95% CI, 1.43-4.99]). SARS-CoV-2-positive children were more likely to report PCCs at 90 days compared with those who tested negative, both among those who were not hospitalized (55 of 1295 [4.2%; 95% CI, 3.2%-5.5%] vs 35 of 1321 [2.7%; 95% CI, 1.9%-3.7%]; difference, 1.6% [95% CI, 0.2%-3.0%]) and those who were hospitalized (40 of 391 [10.2%; 95% CI, 7.4%-13.7%] vs 19 of 380 [5.0%; 95% CI, 3.0%-7.7%]; difference, 5.2% [95% CI, 1.5%-9.1%]). In addition, SARS-CoV-2 positivity was associated with reporting PCCs 90 days after the index ED visit (aOR, 1.63 [95% CI, 1.14-2.35]), specifically systemic health problems (eg, fatigue, weakness, fever; aOR, 2.44 [95% CI, 1.19-5.00]).
Conclusions and relevance: In this cohort study, SARS-CoV-2 infection was associated with reporting PCCs at 90 days in children. Guidance and follow-up are particularly necessary for hospitalized children who have numerous acute symptoms and are older.
Conflict of interest statement
Figures
References
-
- World Health Organization . Expanding our understanding of post COVID-19 condition: report of a WHO webinar—9 February 2021. Accessed May 24, 2022. https://www.who.int/publications/i/item/9789240025035
-
- Centers for Disease Control and Prevention . Post-COVID conditions: information for healthcare providers. Accessed August 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-c...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

