Clinical and radiographic outcomes following transcrestal maxillary sinus floor elevation with injectable xenogenous bone substitute in gel form: a prospective multicenter study
- PMID: 35867239
- PMCID: PMC9307698
- DOI: 10.1186/s40729-022-00431-5
Clinical and radiographic outcomes following transcrestal maxillary sinus floor elevation with injectable xenogenous bone substitute in gel form: a prospective multicenter study
Abstract
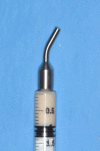
Purpose: To investigate clinical and radiographic outcomes of transcrestal maxillary sinus floor elevation performed with an injectable xenograft in gel form, analyzing general, local and surgical variables possibly influencing the results.
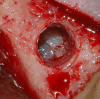
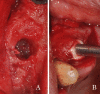
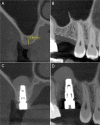
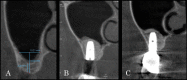
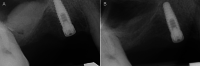
Methods: Patients with residual crestal height < 5 mm underwent transcrestal sinus floor elevation with xenograft in gel form to allow the placement of a single implant. Simultaneous implant placement was performed when primary stability was ≥ 15 Ncm. Graft height was measured immediately after surgery (T0) and after 6 months of healing (T1). Univariate and multivariate regression models were built to assess associations between clinical variables with implant survival and graft height at T1.
Results: 71 patients underwent transcrestal sinus floor elevation and 54 implants were simultaneously placed. Delayed implant placement (at T1) was possible in 5 cases out of 17 (29.4%), whereas in 12 patients (70.6%) implant insertion was not possible or required additional sinus grafting. Implant survival rate, with a follow-up varying from 12 to 32 months after loading, was 100%. Mean pre-operative bone height was 3.8 ± 1.0 mm, at T0 was 13.9 ± 2.2 mm and at T1 was 9.9 ± 2.8 mm. Bone height at T1 was negatively influenced by membrane perforation at surgery (p = 0.004) and positively influenced by immediate implant insertion (p < 0.001).
Conclusions: Transcrestal sinus floor elevation performed with injectable xenograft gel resulted in 100% implant survival rate. However, immediate implant insertion seems a crucial factor to preserve vertical bone gain: one-stage technique seems to be the most predictable approach to optimize clinical outcomes with this approach. Trial registration clinicaltrials.gov, NCT05305521. Registered 31 March 2022-Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT05305521 .
Keywords: Gel graft; Maxillary sinus augmentation; Transcrestal approach.
© 2022. The Author(s).
Conflict of interest statement
All authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Transcrestal maxillary sinus floor elevation with injectable xenogeneic bone substitute in gel form: A clinical, radiological and histological analysis.Am J Dent. 2024 Sept;37(SIA):25A-28A. Am J Dent. 2024. PMID: 39374508 Clinical Trial.
-
Antral membrane balloon technique versus Densah bur in crestal sinus lift with simultaneous implant placement: a randomized clinical trial.BMC Oral Health. 2024 Aug 8;24(1):916. doi: 10.1186/s12903-024-04609-8. BMC Oral Health. 2024. PMID: 39118095 Free PMC article. Clinical Trial.
-
Intraoperative complications and early implant failure after transcrestal sinus floor elevation with residual bone height ≤5 mm: A retrospective multicenter study.Clin Oral Implants Res. 2022 Aug;33(8):783-791. doi: 10.1111/clr.13959. Epub 2022 May 29. Clin Oral Implants Res. 2022. PMID: 35578774 Free PMC article.
-
Graft-Free Maxillary Sinus Floor Elevation: A Systematic Review and Meta-Analysis.J Periodontol. 2017 Jun;88(6):550-564. doi: 10.1902/jop.2017.160665. Epub 2017 Feb 7. J Periodontol. 2017. PMID: 28168901
-
Clinical and radiographic changes following transcrestal sinus augmentation: A scoping review of the last 25 years.Clin Implant Dent Relat Res. 2024 Dec;26(6):1338-1353. doi: 10.1111/cid.13389. Epub 2024 Sep 30. Clin Implant Dent Relat Res. 2024. PMID: 39350529 Free PMC article.
Cited by
-
Prevention and management of intra-operative complications in maxillary sinus augmentation: A review.Clin Implant Dent Relat Res. 2025 Feb;27(1):e13397. doi: 10.1111/cid.13397. Epub 2024 Oct 8. Clin Implant Dent Relat Res. 2025. PMID: 39379340 Free PMC article. Review.
-
Clinical Indications and Outcomes of Sinus Floor Augmentation With Bone Substitutes: An Evidence-Based Review.Clin Implant Dent Relat Res. 2025 Feb;27(1):e13400. doi: 10.1111/cid.13400. Epub 2024 Oct 17. Clin Implant Dent Relat Res. 2025. PMID: 39415739 Free PMC article. Review. No abstract available.
-
Biomechanical Basis for Bone Healing and Osseointegration of Implants in Sinus Grafts.Clin Implant Dent Relat Res. 2025 Feb;27(1):e13424. doi: 10.1111/cid.13424. Epub 2024 Dec 5. Clin Implant Dent Relat Res. 2025. PMID: 39637842 Free PMC article. Review.
References
-
- Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodont Restor Dent. 2003;23:313–323. - PubMed
-
- Lombardi T, Bernardello F, Berton F, Porrelli D, Rapani A, Camurri Piloni A, et al. Efficacy of alveolar ridge preservation after maxillary molar extraction in reducing crestal bone resorption and sinus pneumatization: a multicenter prospective case-control study. Biomed Res Int. 2018;2018:9352130. doi: 10.1155/2018/9352130. - DOI - PMC - PubMed
-
- Pramstraller M, Farina R, Franceschetti G, Pramstraller C, Trombelli L. Ridge dimensions of the edentulous posterior maxilla: a retrospective analysis of a cohort of 127 patients using computerized tomography data. Clin Oral Implants Res. 2011;22:54–61. doi: 10.1111/j.1600-0501.2010.01984.x. - DOI - PubMed
-
- Katsoulis J, Enkling N, Takeichi T, Urban IA, Mericske-Stern R, Avrampou M. Relative bone width of the edentulous maxillary ridge. Clinical implications of digital assessment in presurgical implant planning. Clin Implant Dent Relat Res. 2012;14:213–223. doi: 10.1111/j.1708-8208.2012.00441.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

