Intracellular signaling control of mechanical homeostasis in the aorta
- PMID: 35867282
- PMCID: PMC10547132
- DOI: 10.1007/s10237-022-01593-2
Intracellular signaling control of mechanical homeostasis in the aorta
Abstract
Mature arteries exhibit a preferred biomechanical state in health evidenced by a narrow range of intramural and wall shear stresses. When stresses are perturbed by changes in blood pressure or flow, homeostatic mechanisms tend to restore target values via altered contractility and/or cell and matrix turnover. In contrast, vascular disease associates with compromised homeostasis, hence we must understand mechanisms underlying mechanical homeostasis and its robustness. Here, we use a multiscale computational model wherein mechanosensitive intracellular signaling pathways drive arterial growth and remodeling. First, we identify an ensemble of cell-level parameterizations where tissue-level responses are well-regulated and adaptive to hemodynamic perturbations. The responsible mechanism is persistent multiscale negative feedback whereby mechanosensitive signaling drives mass turnover until homeostatic target stresses are reached. This demonstrates how robustness emerges despite inevitable cell and individual heterogeneity. Second, we investigate tissue-level effects of signaling node knockdowns (ATIR, ROCK, TGF[Formula: see text]RII, PDGFR, ERK1/2) and find general agreement with experimental reports of fault tolerance. Robustness against structural changes manifests via low engagement of the node under baseline stresses or compensatory multiscale feedback via upregulation of additional pathways. Third, we show how knockdowns affect collagen and smooth muscle turnover at baseline and with perturbed stresses. In several cases, basal production is not remarkably affected, but sensitivities to stress deviations, which influence feedback strength, are reduced. Such reductions can impair adaptive responses, consistent with previously reported aortic vulnerability despite grossly normal appearances. Reduced stress sensitivities thus form a candidate mechanism for how robustness is lost, enabling transitions from health towards disease.
Keywords: Growth and remodeling; Homeostasis; Mechanobiology; Multiscale; Robustness.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures
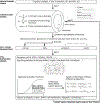




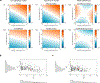
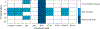
References
-
- Bellini C, Bersi MR, Caulk AW, Ferruzzi J, Milewicz DM, Ramirez F, Rifkin DB, Tellides G, Yanagisawa H, and Humphrey JD. 2017. Comparison of 10 murine models reveals a distinct biomechanical phenotype in thoracic aortic aneurysms. Journal of The Royal Society Interface 14 (130): 20161036. - PMC - PubMed
-
- Cao Z, Liao Q, Su M, Huang K, Jin J, and Cao D. 2019. Akt and erk dual inhibitors: The way forward? Cancer letters 459: 30–40. - PubMed
-
- Chien S 2007. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. American Journal of Physiology-Heart and Circulatory Physiology 292 (3): H1209–H1224. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

