Intact HIV Proviruses Persist in the Brain Despite Viral Suppression with ART
- PMID: 35867351
- PMCID: PMC9489665
- DOI: 10.1002/ana.26456
Intact HIV Proviruses Persist in the Brain Despite Viral Suppression with ART
Abstract
Objective: Human immunodeficiency virus (HIV) persistence in blood and tissue reservoirs, including the brain, is a major barrier to HIV cure and possible cause of comorbid disease. However, the size and replication competent nature of the central nervous system (CNS) reservoir is unclear. Here, we used the intact proviral DNA assay (IPDA) to provide the first quantitative assessment of the intact and defective HIV reservoir in the brain of people with HIV (PWH).
Methods: Total, intact, and defective HIV proviruses were measured in autopsy frontal lobe tissue from viremic (n = 18) or virologically suppressed (n = 12) PWH. Total or intact/defective proviruses were measured by detection of HIV pol or the IPDA, respectively, through use of droplet digital polymerase chain reaction (ddPCR). HIV-seronegative individuals were included as controls (n = 6).
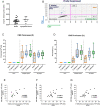
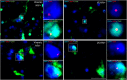
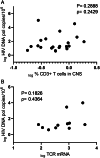
Results: Total HIV DNA was present at similar levels in brain tissues from untreated viremic and antiretroviral (ART)-suppressed individuals (median = 22.3 vs 26.2 HIV pol copies/106 cells), reflecting a stable CNS reservoir of HIV that persists despite therapy. Furthermore, 8 of 10 viremic and 6 of 9 virally suppressed PWH also harbored intact proviruses in the CNS (4.63 vs 12.7 intact copies/106 cells). Viral reservoirs in CNS and matched lymphoid tissue were similar in the composition of intact and/or defective proviruses, albeit at lower levels in the brain. Importantly, CNS resident CD68+ myeloid cells in virally suppressed individuals harbored HIV DNA, directly showing the presence of a CNS resident HIV reservoir.
Interpretation: Our results demonstrate the first evidence for an intact, potentially replication competent HIV reservoir in the CNS of virally suppressed PWH. ANN NEUROL 2022;92:532-544.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
S.R.L. has received investigator‐initiated grant funding from Gilead, Merck, and ViiV Healthcare. She has provided paid scientific advice to Abivax, Abbvie, and Gilead. P.R.G. previously received investigator‐initiated grant funding from ViiV Healthcare. <zbmrule>
Figures




References
-
- Churchill MJ, Gorry PR, Cowley D, et al. Use of laser capture microdissection to detect integrated HIV‐1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol 2006;12:146–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

