Detecting Waning Serological Response with Commercial Immunoassays: 18-Month Longitudinal Follow-up of Anti-SARS-CoV-2 Nucleocapsid Antibodies
- PMID: 35867423
- PMCID: PMC9430644
- DOI: 10.1128/spectrum.00986-22
Detecting Waning Serological Response with Commercial Immunoassays: 18-Month Longitudinal Follow-up of Anti-SARS-CoV-2 Nucleocapsid Antibodies
Abstract
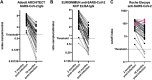
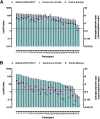
Past severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is an important determinant of protection from reinfection and of postvaccine immune responses. Herein, we conducted a follow-up analysis of health care workers previously infected with coronavirus disease 2019 (COVID-19) with the aim of evaluating different immunoassays for their capability in detecting the waning anti-SARS-CoV-2 immune responses and accuracy in documenting past SARS-CoV-2 infections. We evaluated serum antinucleocapsid antibody levels in convalescent individuals following a 1.5-year interval from SARS-CoV-2 infection. Three different commercial immunoassays that qualitatively measure serum antibodies targeting the SARS-CoV-2 nucleocapsid protein, namely, the Abbott Architect SARS-CoV-2 IgG, the Euroimmun anti-SARS-CoV-2 NCP enzyme-linked immunosorbent assay (ELISA) IgG, and the Roche Elecsys anti-SARS-CoV-2, were tested for comparison of detectability. A total of 38 individuals consented to participating in this follow-up analysis. From assay to assay, seropositivity rate at 18 months from infection varied from lowest at 42% to highest at 92%. The Roche Elecsys immunoassay, dependent on the dual-antigen antibody detection method and tuned for the detection of high avidity antibodies, was most capable of accurately documenting past SARS-CoV-2 infections. Different immunoassays showed variable capability of determining previous infection status under waning antibody concentrations. Immunoassays with lower detection limits are to be selected, and adjusted thresholds are to be considered in order to maximize the tests' performance. IMPORTANCE Past SARS-CoV-2 infection is an important determinant of protection from reinfection and of postvaccine immune responses. Our results show that different immunoassays, by design, harbor variable capability of tracking SARS-CoV-2 infection under waning antibody concentrations. With each recovered patient standing at a unique time point along the decline curve of antibodies, precise estimation of COVID-19 cumulative incidence remains a challenge. Since future surveillance studies will be targeting more than ever heterogenous cohorts, selecting the appropriate immunoassay is crucial in order to assure reliable decisions about an individual's previous infection status.
Keywords: COVID-19; SARS-CoV-2; serological kinetics; waning antibody.
Conflict of interest statement
The authors declare a conflict of interest. This research was supported by Japan Agency for Medical Research and Development [JP20jk0110021] and Osaka City University Strategic Research Grant [OCU-SRG2021_YR09]. Yu Nakagama and Yasutoshi Kido receive financial support from Abbott Japan LLC, Japan, outside the work.
Figures
Similar articles
-
SARS-CoV-2 Antibody Testing in Health Care Workers: A Comparison of the Clinical Performance of Three Commercially Available Antibody Assays.Microbiol Spectr. 2021 Oct 31;9(2):e0039121. doi: 10.1128/Spectrum.00391-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585976 Free PMC article.
-
Concordance of SARS-CoV-2 Antibody Results during a Period of Low Prevalence.mSphere. 2022 Oct 26;7(5):e0025722. doi: 10.1128/msphere.00257-22. Epub 2022 Sep 29. mSphere. 2022. PMID: 36173112 Free PMC article.
-
Assessment of S1-, S2-, and NCP-Specific IgM, IgA, and IgG Antibody Kinetics in Acute SARS-CoV-2 Infection by a Microarray and Twelve Other Immunoassays.J Clin Microbiol. 2021 Apr 20;59(5):e02890-20. doi: 10.1128/JCM.02890-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33602698 Free PMC article.
-
Performance of Elecsys Anti-SARS CoV-2 (Roche) and VIDAS Anti-SARS CoV-2 (Biomérieux) for SARS-CoV-2 Nucleocapsid and Spike Protein Antibody Detection.EJIFCC. 2022 Aug 8;33(2):159-165. eCollection 2022 Aug. EJIFCC. 2022. PMID: 36313907 Free PMC article. Review.
-
Humoral immunological kinetics of severe acute respiratory syndrome coronavirus 2 infection and diagnostic performance of serological assays for coronavirus disease 2019: an analysis of global reports.Int Health. 2022 Jan 19;14(1):18-52. doi: 10.1093/inthealth/ihab005. Int Health. 2022. PMID: 33620427 Free PMC article. Review.
Cited by
-
Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa.Nat Commun. 2023 Jan 16;14(1):246. doi: 10.1038/s41467-022-35652-0. Nat Commun. 2023. PMID: 36646700 Free PMC article.
-
Korea Seroprevalence Study of Monitoring of SARS-COV-2 Antibody Retention and Transmission (K-SEROSMART): findings from national representative sample.Epidemiol Health. 2023;45:e2023075. doi: 10.4178/epih.e2023075. Epub 2023 Aug 17. Epidemiol Health. 2023. PMID: 37591786 Free PMC article.
-
Sustained spike-specific IgG antibodies following CoronaVac (Sinovac) vaccination in sub-Saharan Africa, but increased breakthrough infections in baseline spike-naive individuals.Front Immunol. 2023 Nov 30;14:1255676. doi: 10.3389/fimmu.2023.1255676. eCollection 2023. Front Immunol. 2023. PMID: 38098482 Free PMC article.
-
Ethnic differences in cellular and humoral immune responses to SARS-CoV-2 vaccination in UK healthcare workers: a cross-sectional analysis.EClinicalMedicine. 2023 Apr;58:101926. doi: 10.1016/j.eclinm.2023.101926. Epub 2023 Apr 4. EClinicalMedicine. 2023. PMID: 37034357 Free PMC article.
-
Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa.medRxiv [Preprint]. 2022 Aug 22:2022.08.19.22278993. doi: 10.1101/2022.08.19.22278993. medRxiv. 2022. Update in: Nat Commun. 2023 Jan 16;14(1):246. doi: 10.1038/s41467-022-35652-0. PMID: 36032973 Free PMC article. Updated. Preprint.
References
-
- Ontañón J, Blas J, de Cabo C, Santos C, Ruiz-Escribano E, García A, Marín L, Sáez L, Beato JL, Rada R, Navarro L, Sainz de Baranda C, Solera J. 2021. Influence of past infection with SARS-CoV-2 on the response to the BNT162b2 mRNA vaccine in health care workers: kinetics and durability of the humoral immune response. EBioMedicine 73:103656. doi:10.1016/j.ebiom.2021.103656. - DOI - PMC - PubMed
-
- Nakagama Y, Komase Y, Candray K, Nakagama S, Sano F, Tsuchida T, Kunishima H, Imai T, Shintani A, Nitahara Y, Kaku N, Kido Y. 2021. Serological testing reveals the hidden COVID-19 burden among health care workers experiencing a SARS-CoV-2 nosocomial outbreak. Microbiol Spectr 9:e01082-21. doi:10.1128/Spectrum.01082-21. - DOI - PMC - PubMed
-
- Nakagama Y, Rodriguez-Funes MV, Dominguez R, Candray-Medina KS, Uemura N, Tshibangu-Kabamba E, Nitahara Y, Kaku N, Kaneko A, Kido Y. 2022. Cumulative seroprevalence among healthcare workers after the first wave of the COVID-19 pandemic in El Salvador, Central America. medRxiv. doi:10.1101/2022.02.06.22270565. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

