Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru
- PMID: 35867471
- PMCID: PMC9430815
- DOI: 10.1128/spectrum.00861-22
Diagnostic Performance Assessment of Saliva RT-PCR and Nasopharyngeal Antigen for the Detection of SARS-CoV-2 in Peru
Abstract
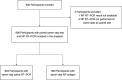
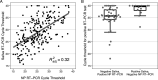
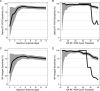
Widely available and reliable testing for SARS-CoV-2 is essential for the public health response to the COVID-19 pandemic. We estimated the diagnostic performance of reverse transcription PCR (RT-PCR) performed on saliva and the SD Biosensor STANDARD Q antigen test performed on nasopharyngeal swab compared to the reference standard, nasopharyngeal swab (NP) RT-PCR. We enrolled participants living and/or seeking care in health facilities in North Lima, Peru from November 2020 to January 2021. Consenting participants underwent same-day RT-PCR on both saliva and nasopharyngeal swab specimens, antigen testing on a nasopharyngeal swab specimen, pulse oximetry, and standardized symptom assessment. We calculated sensitivity, specificity, and predictive values for the nasopharyngeal antigen and saliva RT-PCR compared to nasopharyngeal RT-PCR. Of 896 participants analyzed, 567 (63.3%) had acute signs/symptoms of COVID-19. The overall sensitivity and specificity of saliva RT-PCR were 85.8% and 98.1%, respectively. Among participants with and without acute signs/symptoms of COVID-19, saliva sensitivity was 87.3% and 37.5%, respectively. Saliva sensitivity was 97.4% and 56.0% among participants with cycle threshold (CT) values of ≤30 and >30 on nasopharyngeal RT-PCR, respectively. The overall sensitivity and specificity of nasopharyngeal antigen were 73.2% and 99.4%, respectively. The sensitivity of the nasopharyngeal antigen test was 75.1% and 12.5% among participants with and without acute signs/symptoms of COVID-19, and 91.2% and 26.7% among participants with CT values of ≤30 and >30 on nasopharyngeal RT-PCR, respectively. Saliva RT-PCR achieved the WHO-recommended threshold of >80% for sensitivity for the detection of SARS-CoV-2, while the SD Biosensor nasopharyngeal antigen test did not. IMPORTANCE In this diagnostic validation study of 896 participants in Peru, saliva reverse transcription PCR (RT-PCR) had >80% sensitivity for the detection of SARS-CoV-2 among all-comers and symptomatic individuals, while the SD Biosensor STANDARD Q antigen test performed on nasopharyngeal swab had <80% sensitivity, except for participants whose same-day nasopharyngeal RT-PCR results showed cycle threshold values of <30, consistent with a high viral load in the nasopharynx. The specificity was high for both tests. Our results demonstrate that saliva sampling could serve as an alternative noninvasive technique for RT-PCR diagnosis of SARS-CoV-2. The role of nasopharyngeal antigen testing is more limited; when community transmission is low, it may be used for mass screenings among asymptomatic individuals with high testing frequency. Among symptomatic individuals, the nasopharyngeal antigen test may be relied upon for 4 to 8 days after symptom onset, or in those likely to have high viral load, whereupon it showed >80% sensitivity.
Keywords: COVID-19; Peru; RT-PCR; SARS-CoV-2; antigen; diagnosis; nasopharyngeal swab; saliva; validation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Diagnostic Performance and Tolerability of Saliva and Nasopharyngeal Swab Specimens in the Detection of SARS-CoV-2 by RT-PCR.Microbiol Spectr. 2023 Jun 15;11(3):e0532422. doi: 10.1128/spectrum.05324-22. Epub 2023 Apr 24. Microbiol Spectr. 2023. PMID: 37093085 Free PMC article.
-
Comparison between Nasal and Nasopharyngeal Swabs for SARS-CoV-2 Rapid Antigen Detection in an Asymptomatic Population, and Direct Confirmation by RT-PCR from the Residual Buffer.Microbiol Spectr. 2022 Feb 23;10(1):e0245521. doi: 10.1128/spectrum.02455-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171010 Free PMC article.
-
SARS-CoV-2 saliva testing using RT-PCR: a systematic review.Int J Infect Dis. 2022 Aug;121:166-171. doi: 10.1016/j.ijid.2022.05.008. Epub 2022 May 13. Int J Infect Dis. 2022. PMID: 35577250 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
Cited by
-
Laboratory Evaluation Links Some False-Positive COVID-19 Antigen Test Results Observed in a Field Study to a Specific Lot of Test Strips.Open Forum Infect Dis. 2023 Jan 5;10(1):ofac701. doi: 10.1093/ofid/ofac701. eCollection 2023 Jan. Open Forum Infect Dis. 2023. PMID: 36726541 Free PMC article.
-
Recent advances in RNA sample preparation techniques for the detection of SARS-CoV-2 in saliva and gargle.Trends Analyt Chem. 2023 Aug;165:117107. doi: 10.1016/j.trac.2023.117107. Epub 2023 May 23. Trends Analyt Chem. 2023. PMID: 37317683 Free PMC article. Review.
References
-
- WHO. 2021. WHO COVID-19 dashboard. WHO, Geneva, Switzerland. https://covid19.who.int. Accessed 26 August 2021.
-
- WHO. 2021. Considerations for implementing and adjusting public health and social measures in the context of COVID-19. WHO, Geneva, Switzerland. https://www.who.int/publications/i/item/considerations-in-adjusting-publ.... Accessed 26 August 2021.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

