Conserved Lysine Residues of Hepatitis B Virus Core Protein Are Not Required for Covalently Closed Circular DNA Formation
- PMID: 35867543
- PMCID: PMC9364803
- DOI: 10.1128/jvi.00718-22
Conserved Lysine Residues of Hepatitis B Virus Core Protein Are Not Required for Covalently Closed Circular DNA Formation
Abstract
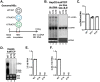
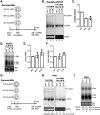
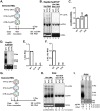
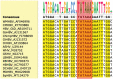
Hepatitis B virus (HBV) core protein (HBc), the building block of the viral capsid, plays a critical role throughout the HBV life cycle. There are two highly conserved lysine residues, namely, K7 and K96, on HBc, which have been proposed to function at various stages of viral replication, potentially through lysine-specific posttranslational modifications (PTMs). Here, we substituted K7 and K96 with alanine or arginine, which would also block potential PTMs on these two lysine residues, and tested the effects of these substitutions on HBV replication and infection. We found that the two lysine residues were dispensable for all intracellular steps of HBV replication. In particular, all mutants were competent to form the covalently closed circular DNA (cccDNA) via the intracellular amplification pathway, indicating that K7 and K96, or any PTMs of these residues, were not essential for nucleocapsid uncoating, a prerequisite for cccDNA formation. Furthermore, we found that K7A and K7R mutations did not affect de novo cccDNA formation and RNA transcription during infection, indicating that K7 or any PTMs of this residue were dispensable for HBV infection. In addition, we demonstrated that the HBc K7 coding sequence (AAA), as part of the HBV polyadenylation signal UAUAAA, was indispensable for viral RNA production, implicating this cis requirement at the RNA level, instead of any function of HBc-K7, likely constrains the identity of the 7th residue of HBc. In conclusion, our results provided novel insights regarding the roles of lysine residues on HBc, and their coding sequences, in the HBV life cycle. IMPORTANCE Hepatitis B virus (HBV) infection remains a public health burden that affects 296 million individuals worldwide. HBV core protein (HBc) is involved in almost all steps in the HBV life cycle. There are two conserved lysine residues on HBc. Here, we found that neither of them is essential for HBV intracellular replication, including the formation of covalently closed circular DNA (cccDNA), the molecular basis for establishing and sustaining the HBV infection. However, K96 is critical for virion morphogenesis, while the K7 coding sequence, but not HBc-K7 itself, is indispensable, as part of the RNA polyadenylation signal, for HBV RNA production from cccDNA. Our results provide novel insights regarding the role of the conserved lysine residues on HBc, and their coding sequences, in viral replication, and should facilitate the development of antiviral drugs against the HBV capsid protein.
Keywords: capsid; cccDNA; core; hepatitis B virus; infection; lysine; polyadenylation; posttranslational modification; replication; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

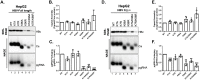
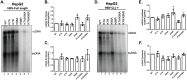
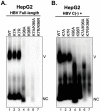
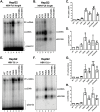
Similar articles
-
Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation.Viruses. 2023 Feb 28;15(3):642. doi: 10.3390/v15030642. Viruses. 2023. PMID: 36992351 Free PMC article. Review.
-
Hepatitis B Virus Core Protein Is Not Required for Covalently Closed Circular DNA Transcriptional Regulation.J Virol. 2022 Nov 9;96(21):e0136222. doi: 10.1128/jvi.01362-22. Epub 2022 Oct 13. J Virol. 2022. PMID: 36226986 Free PMC article.
-
Region-Specific Hepatitis B Virus Genome Exposure from Nucleocapsid Modulated by Capsid Linker Sequence and Inhibitor: Implications for Uncoating.J Virol. 2022 Apr 27;96(8):e0039922. doi: 10.1128/jvi.00399-22. Epub 2022 Apr 7. J Virol. 2022. PMID: 35389266 Free PMC article.
-
Pregenomic RNA Launch Hepatitis B Virus Replication System Facilitates the Mechanistic Study of Antiviral Agents and Drug-Resistant Variants on Covalently Closed Circular DNA Synthesis.J Virol. 2022 Dec 21;96(24):e0115022. doi: 10.1128/jvi.01150-22. Epub 2022 Nov 30. J Virol. 2022. PMID: 36448800 Free PMC article.
-
Intracellular Trafficking of HBV Particles.Cells. 2020 Sep 2;9(9):2023. doi: 10.3390/cells9092023. Cells. 2020. PMID: 32887393 Free PMC article. Review.
Cited by
-
SUMO Modification of Hepatitis B Virus Core Mediates Nuclear Entry, Promyelocytic Leukemia Nuclear Body Association, and Efficient Formation of Covalently Closed Circular DNA.Microbiol Spectr. 2023 Jun 15;11(3):e0044623. doi: 10.1128/spectrum.00446-23. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199632 Free PMC article.
-
NEDD4 family ubiquitin ligase AIP4 interacts with Alix to enable HBV naked capsid egress in an Alix ubiquitination-independent manner.PLoS Pathog. 2024 Sep 11;20(9):e1012485. doi: 10.1371/journal.ppat.1012485. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39259704 Free PMC article.
-
Hepatitis B Virus Capsid: The Core in Productive Entry and Covalently Closed Circular DNA Formation.Viruses. 2023 Feb 28;15(3):642. doi: 10.3390/v15030642. Viruses. 2023. PMID: 36992351 Free PMC article. Review.
-
Characterization of Intracellular Precore-Derived Proteins and Their Functions in Hepatitis B Virus-Infected Human Hepatocytes.mBio. 2023 Feb 28;14(1):e0350122. doi: 10.1128/mbio.03501-22. Epub 2023 Jan 30. mBio. 2023. PMID: 36715515 Free PMC article.
-
Detection of Hepatitis B Virus Covalently Closed Circular DNA and Intermediates in Its Formation.Methods Mol Biol. 2024;2837:99-111. doi: 10.1007/978-1-0716-4027-2_9. Methods Mol Biol. 2024. PMID: 39044078 Free PMC article.
References
-
- WHO. 2021. Hepatitis B. WHO, Geneva, Switzerland. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 1:e00049. 10.7554/eLife.00049. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

