Medicaid and Accelerated Approval: Spending on Drugs with and without Proven Clinical Benefits
- PMID: 35867545
- PMCID: PMC9789165
- DOI: 10.1215/03616878-10041107
Medicaid and Accelerated Approval: Spending on Drugs with and without Proven Clinical Benefits
Abstract
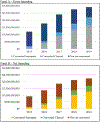
Many state Medicaid officials are concerned about rising prescription drug spending, particularly drugs approved through the Food and Drug Administration's (FDA) accelerated approval pathway. The authors examined how much of Medicaid programs' accelerated approval spending is attributable to products that have demonstrated clinical benefits versus those that have not. Their findings provide support for states' concerns that pharmaceutical companies often fail to complete their required postapproval confirmatory studies within the FDA's requested timeline. But the findings also highlight one issue that policy stakeholders have not yet devoted substantial attention to: the use of surrogate endpoints involved in the postapproval confirmatory studies for most of the products in this study's sample. The granularity of the study's results enabled an analysis of the impact of different policy recommendations on both the accelerated approval pathway and Medicaid programs. These findings inform the current policy debate, suggesting that policy stakeholders might focus attention on products converting their approval on the basis of surrogate outcomes rather than on clinical outcomes.
Keywords: Food and Drug Administration; Medicaid; accelerated approval; drug pricing.
Copyright © 2022 by Duke University Press.
Figures

References
-
- CMS (Centers for Medicare & Medicaid Services). 2018. “State Medicaid Coverage of Drugs Approved by the FDA Under Accelerated Approval Pathway.” https://www.medicaid.gov/medicaid-chip-program-information/by-topics/pre....
-
- CMS (Centers for Medicare & Medicaid Services). 2021. “State Drug Utilization Data.” https://www.medicaid.gov/medicaid/prescription-drugs/state-drug-utilizat....
-
- CBO (Congressional Budget Office). 2021. “A Comparison of Brand-Name Drug Prices Among Selected Federal Programs.” https://www.cbo.gov/publication/56978.
-
- FDA (Food & Drug Administration). 2015. “Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral Drugs for Treatment: Guidance for Industry.” https://www.fda.gov/media/86284/download.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

