Establishment, Persistence, and Reactivation of Latent HIV-1 Infection in Renal Epithelial Cells
- PMID: 35867560
- PMCID: PMC9327708
- DOI: 10.1128/jvi.00624-22
Establishment, Persistence, and Reactivation of Latent HIV-1 Infection in Renal Epithelial Cells
Abstract
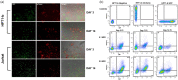
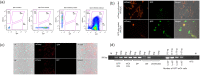
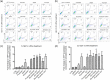
HIV-1 persistence in different cell types presents the main obstacle to an HIV-1 cure. We have previously shown that the renal epithelium is a site of HIV-1 infection and that the kidney represents a separate viral compartment from blood. Whether renal cells can harbor latent virus that can be reactivated upon treatment with latency reversing agents (LRAs) is unknown. To address this question, we developed an in vitro HIV-1 latency model in renal tubule epithelial (RTE) cells using a dual color HIV-1 reporter virus, R7/E-/GFP/EF1a-mCherry (R7GEmC), and evaluated the effect of LRAs, both as single agents and in combination, on viral reactivation. Our data show that HIV-1 can establish latency in RTE cells early postinfection. While the pool of latently infected cells expanded overtime, the percentage of productively infected cells declined. Following LRA treatment only a small fraction of latently infected cells, both T cells and RTE cells, could be reactivated, and the drug combinations more effective in reactivating HIV transcription in RTE cells differed from those more active in T cells. Our study demonstrates that HIV can establish latency in RTE cells and that current LRAs are only marginally effective in inducing HIV-1 reactivation. This suggests that further study of LRA dynamics in non-T cells may be warranted to assess the suitability of LRAs as a sterilizing cure strategy. IMPORTANCE Anti-retroviral therapy (ART) has dramatically reduced HIV-related morbidity and mortality. Despite this success, a number of challenges remain, including the long-term persistence of multiple, clinically latent viral reservoirs capable of reactivation in the absence of ART. As efforts proceed toward HIV eradication or functional cure, further understanding of the dynamics of HIV-1 replication, establishment of latency and mechanisms of reactivation in reservoirs harboring the virus throughout the body is necessary. HIV-1 can infect renal epithelial cells and the expression of viral genes in those cells contributes to the development of HIV associated nephropathy (HIVAN) in untreated individuals. The significance of our work is in developing the first model of HIV-1 latency in renal epithelial cells. This model enhances our understanding of HIV-1 latency and persistence in the kidney and can be used to screen candidate latency reversing agents.
Keywords: HIV-1; LRAs; latency; renal epithelial cells; reservoir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Alternate NF-κB-Independent Signaling Reactivation of Latent HIV-1 Provirus.J Virol. 2019 Aug 28;93(18):e00495-19. doi: 10.1128/JVI.00495-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243131 Free PMC article.
-
Activation of Latent HIV-1 T Cell Reservoirs with a Combination of Innate Immune and Epigenetic Regulators.J Virol. 2019 Oct 15;93(21):e01194-19. doi: 10.1128/JVI.01194-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413127 Free PMC article.
-
Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy.J Virol. 2016 Oct 14;90(21):9712-9724. doi: 10.1128/JVI.00852-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535056 Free PMC article.
-
HIV Tat as a latency reversing agent: turning the tables on viral persistence.Front Immunol. 2025 Apr 11;16:1571151. doi: 10.3389/fimmu.2025.1571151. eCollection 2025. Front Immunol. 2025. PMID: 40292298 Free PMC article. Review.
-
A Canadian Survey of Research on HIV-1 Latency-Where Are We Now and Where Are We Heading?Viruses. 2024 Feb 1;16(2):229. doi: 10.3390/v16020229. Viruses. 2024. PMID: 38400005 Free PMC article. Review.
Cited by
-
HIV-1 infection of renal epithelial cells: 30 years of evidence from transgenic animal models, human studies and in vitro experiments.Retrovirology. 2023 Mar 16;20(1):2. doi: 10.1186/s12977-023-00617-8. Retrovirology. 2023. PMID: 36927552 Free PMC article. Review.
-
Cardiac and Renal Comorbidities in Aging People Living With HIV.Circ Res. 2024 May 24;134(11):1636-1660. doi: 10.1161/CIRCRESAHA.124.323948. Epub 2024 May 23. Circ Res. 2024. PMID: 38781295 Free PMC article. Review.
References
-
- Deeks SG, Lewin SR, Ross AL, Ananworanich J, Benkirane M, Cannon P, Chomont N, Douek D, Lifson JD, Lo Y-R, Kuritzkes D, Margolis D, Mellors J, Persaud D, Tucker JD, Barre-Sinoussi F, Alter G, Auerbach J, Autran B, Barouch DH, Behrens G, Cavazzana M, Chen Z, Cohen ÉA, Corbelli GM, Eholié S, Eyal N, Fidler S, Garcia L, Grossman C, Henderson G, Henrich TJ, Jefferys R, Kiem H-P, McCune J, Moodley K, Newman PA, Nijhuis M, Nsubuga MS, Ott M, Palmer S, Richman D, Saez-Cirion A, Sharp M, Siliciano J, Silvestri G, Singh J, Spire B, Taylor J, Tolstrup M, International AIDS Society Towards a Cure Working Group, et al. . 2016. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat Med 22:839–850. 10.1038/nm.4108. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

