Tembusu Virus Nonstructural Protein 2B Antagonizes Type I Interferon Production by Targeting MAVS for Degradation
- PMID: 35867574
- PMCID: PMC9327690
- DOI: 10.1128/jvi.00816-22
Tembusu Virus Nonstructural Protein 2B Antagonizes Type I Interferon Production by Targeting MAVS for Degradation
Abstract
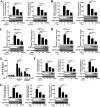
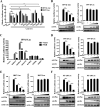
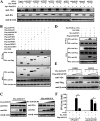
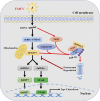
Tembusu virus (TMUV) is a newly emerged avian flavivirus that has caused severe egg-drop syndrome and fatal encephalitis in domestic ducks. It has spread widely throughout the main duck-producing areas in Asia, resulting in substantial economic losses to the duck industry. Previous studies have reported that TMUV has evolved several strategies to counteract the duck's innate immune responses to successfully establish infection in its host cells. However, the mechanisms underlying this phenomenon have not been elucidated. Here, we discovered that TMUV-encoded NS2B is a negative regulator of poly(I:C)-induced duck interferon-β (IFN-β) expression. Mechanistically, TMUV NS2B was found to interact specifically with the mitochondrial antiviral-signaling protein (duMAVS). Consequently, duMAVS was degraded through the K48-linked ubiquitination and proteasomal pathway, leading to the interruption of the RIG-I-like receptor (RLR) signaling. Further analyses also identified K321, K354, K398, and K411 as crucial residues for NS2B-mediated ubiquitination and degradation of duMAVS. Additionally, we demonstrated that NS2B functions by recruiting the E3 ubiquitin ligase duck membrane-associated RING-CH-type finger 5 (duMARCH5) to modify duMAVS via polyubiquitination, blocking the duMAVS-mediated innate immune response and promoting TMUV replication. Taken together, our findings revealed a novel mechanism by which TMUV evades the duck's antiviral innate immune responses. IMPORTANCE Tembusu virus (TMUV), an emerging pathogenic flavivirus, has spread to most duck farming areas in Asia since 2010, causing significant economic losses to the duck industry. Recently, TMUV has expanded its host range and may pose a potential threat to mammals, including humans. Understanding the interaction between TMUV and its host is essential for the development of effective vaccines and therapeutics. Here, we show that NS2B encoded by TMUV inhibits IFN production by interacting with duck MAVS (duMAVS) to mediate ubiquitination and proteasomal degradation. Further studies suggest that the E3 ubiquitin ligase duck membrane-associated RING-CH-type finger 5 (duMARCH5) is recruited by NS2B to mediate proteasomal degradation of duMAVS. As a result, the innate immune response triggered by the RIG-I-like receptor (RLR) is disrupted, facilitating viral replication. Overall, our results reveal a novel mechanism by which TMUV evades host innate immunity and provide new therapeutic strategies to prevent TMUV infection.
Keywords: MAVS; NS2B; Tembusu virus; degradation; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

