Research status and development of microbial induced calcium carbonate mineralization technology
- PMID: 35867666
- PMCID: PMC9334024
- DOI: 10.1371/journal.pone.0271761
Research status and development of microbial induced calcium carbonate mineralization technology
Abstract
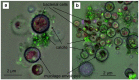
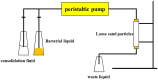
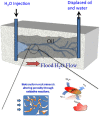
In nature, biomineralization is a common phenomenon, which can be further divided into authigenic and artificially induced mineralization. In recent years, artificially induced mineralization technology has been gradually extended to major engineering fields. Therefore, by elaborating the reaction mechanism and bacteria of mineralization process, and summarized various molecular dynamics equations involved in the mineralization process, including microbial and nutrient transport equations, microbial adsorption equations, growth equations, urea hydrolysis equations, and precipitation equations. Because of the environmental adaptation stage of microorganisms in sandy soil, their reaction rate in sandy soil environment is slower than that in solution environment, the influencing factors are more different, in general, including substrate concentration, temperature, pH, particle size and grouting method. Based on the characteristics of microbial mineralization such as strong cementation ability, fast, efficient, and easy to control, there are good prospects for application in sandy soil curing, building improvement, heavy metal fixation, oil reservoir dissection, and CO2 capture. Finally, it is discussed and summarized the problems and future development directions on the road of commercialization of microbial induced calcium carbonate precipitation technology from laboratory to field application.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Similar articles
-
Preferred injection method and curing mechanism analysis for the curing of loose Pisha sandstone based on microbially induced calcite precipitation.Environ Sci Pollut Res Int. 2023 Jan;30(5):12005-12019. doi: 10.1007/s11356-022-22742-1. Epub 2022 Sep 14. Environ Sci Pollut Res Int. 2023. PMID: 36103070
-
An indigenous bacterium with enhanced performance of microbially-induced Ca-carbonate biomineralization under extreme alkaline conditions for concrete and soil-improvement industries.Acta Biomater. 2021 Jan 15;120:304-317. doi: 10.1016/j.actbio.2020.11.016. Epub 2020 Nov 16. Acta Biomater. 2021. PMID: 33212232
-
Immobilization of Cd2+ and Pb2+ by biomineralization of the carbonate mineralized bacterial consortium JZ1.Environ Sci Pollut Res Int. 2023 Feb;30(9):22471-22482. doi: 10.1007/s11356-022-23587-4. Epub 2022 Oct 27. Environ Sci Pollut Res Int. 2023. PMID: 36301386
-
Remediation of soil cadmium pollution by biomineralization using microbial-induced precipitation: a review.World J Microbiol Biotechnol. 2021 Nov 1;37(12):208. doi: 10.1007/s11274-021-03176-2. World J Microbiol Biotechnol. 2021. PMID: 34719751 Review.
-
A critical review on microbial carbonate precipitation via denitrification process in building materials.Bioengineered. 2021 Dec;12(1):7529-7551. doi: 10.1080/21655979.2021.1979862. Bioengineered. 2021. PMID: 34652267 Free PMC article. Review.
Cited by
-
State-of-the-Art Review on Engineering Uses of Calcium Phosphate Compounds: An Eco-Friendly Approach for Soil Improvement.Materials (Basel). 2022 Oct 3;15(19):6878. doi: 10.3390/ma15196878. Materials (Basel). 2022. PMID: 36234219 Free PMC article. Review.
-
Microbial Carbonate Mineralization: A Comprehensive Review of Mechanisms, Applications, and Recent Advancements.Mol Biotechnol. 2025 May 8. doi: 10.1007/s12033-025-01433-5. Online ahead of print. Mol Biotechnol. 2025. PMID: 40338440 Review.
-
Naturally Derived Cements Learned from the Wisdom of Ancestors: A Literature Review Based on the Experiences of Ancient China, India and Rome.Materials (Basel). 2023 Jan 8;16(2):603. doi: 10.3390/ma16020603. Materials (Basel). 2023. PMID: 36676340 Free PMC article. Review.
-
Single-Particle Crushing Test of Coated Calcareous Sand Based on MICP.Materials (Basel). 2024 Sep 24;17(19):4690. doi: 10.3390/ma17194690. Materials (Basel). 2024. PMID: 39410263 Free PMC article.
-
Effect of Bacillus subtilis on mechanical and self-healing properties in mortar with different crack widths and curing conditions.Sci Rep. 2023 May 15;13(1):7844. doi: 10.1038/s41598-023-34837-x. Sci Rep. 2023. PMID: 37188710 Free PMC article.
References
-
- BOQUET E, BORONAT A, RAMOS—cORMENZANA A. Produc-tion of calcite (Calcium carbonate) crystals by soil bacteria is a gen-eral phenomenon[J]. Nature,1 973,246(5434): 527–529. doi: 10.1038/246527a0 - DOI
-
- WHIFFIN V S. Microbial CaCO3 precipitation for the production of biocement-[D]. Perth, Australia: Murdoch University, 2004.
-
- Mitchell AC, Ferris FG. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: temperature and kinetic dependence. Geochim Cosmochim Acta. 2005;69(17):4199–4210. doi: 10.1016/j.gca.2005.03.014 - DOI
-
- Liu X, Fan J, Yu J, et al.. Solidification of loess using microbial induced carbonate precipitation[J]. Journal of Mountain Science, 2021, 18(1): 265–274.
-
- Imran M A, Kimura S, Nakashima K, et al.. Feasibility study of native ureolytic bacteria for biocementation towards coastal erosion protection by MICP method[J]. Applied Sciences, 2019, 9(20): 4462. doi: 10.3390/app9204462 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

