'Mechanistic insights into 5-lipoxygenase inhibition by active principles derived from essential oils of Curcuma species: Molecular docking, ADMET analysis and molecular dynamic simulation study
- PMID: 35867724
- PMCID: PMC9307165
- DOI: 10.1371/journal.pone.0271956
'Mechanistic insights into 5-lipoxygenase inhibition by active principles derived from essential oils of Curcuma species: Molecular docking, ADMET analysis and molecular dynamic simulation study
Abstract
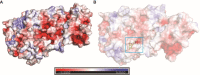
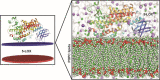
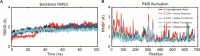
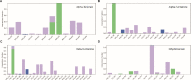
Inflammation is caused by a cascade of events, one of which is the metabolism of arachidonic acid, that begins with oxidation by the enzyme 5-lipoxygenase. 5-Lipoxygenase (5-LOX) plays an important role in the inflammation process by synthesizing leukotrienes and several lipid mediators and has emerged as a possible therapeutic target for treatment of inflammatory diseases such as asthma and rheumatoid arthritis. Most of the existing 5-LOX inhibitors are synthetic and exhibit adverse side effects. In view of this, there is need to search for an alternate source of 5-LOX inhibitor with minimal side effects. The essential oil of several species of Curcuma has received considerable attention in recent times in traditional system of medicine especially for treating various inflammatory disorders. Therefore, the present study was carried out to screen the most potential 5-LOX inhibitors from essential oil components of Curcuma species and elucidate their mechanisms of action through computational biology approaches. Twenty-three phytoconstituents derived from the essential oil of Curcuma species were docked and their predictive binding energies were calculated to select the best possible ligand for 5-LOX. The top 8 ranked compounds from docking was tested for drug-likeness properties, bioactivity score, and toxicity analysis. The phytoconstituents such as α-turmerone, β-turmerone, α-terpineol and dihydrocarveolshowed the best binding affinity with 5-LOX and displayed favorable physicochemical properties. Molecular dynamics simulation in POPC lipid bilayers was carried out to understand the intrinsic dynamics and flexibility of the 5-LOX (apo) and 5-LOX-complex (α-terpineol, α-turmerone, β-turmerone and dihydrocarveol) systems. The molecular dynamic results showed that these 4 phytoconstituents interacted stably with the 5-LOX active site residues and the important bonds that were observed in the initial ligand docked compounds did not alter during the course of simulation. In general, our integrative computational approach demonstrated that the natural compounds like α-turmerone, β-turmerone, α-terpineol, and dihydrocarveol could be considered for designing specific anti-inflammatory drugs using structure-based drug design.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Exploring molecular docking with MM-GBSA and molecular dynamics simulation to predict potent inhibitors of cyclooxygenase (COX-2) enzyme from terpenoid-based active principles of Zingiber species.J Biomol Struct Dyn. 2023 Dec;41(20):10840-10850. doi: 10.1080/07391102.2022.2161011. Epub 2022 Dec 28. J Biomol Struct Dyn. 2023. PMID: 36576262
-
Screening of potent inhibitor from Aquilaria malaccensis Lam. against arachidonic inflammatory enzymes: an insight from molecular docking, ADMET, molecular dynamics simulation and MM-PBSA approaches.J Biomol Struct Dyn. 2024;42(22):12622-12636. doi: 10.1080/07391102.2023.2271977. Epub 2023 Oct 26. J Biomol Struct Dyn. 2024. PMID: 37885259
-
Discovery of natural 15-LOX small molecule inhibitors from Chinese herbal medicine using virtual Screening, biological evaluation and molecular dynamics studies.Bioorg Chem. 2021 Oct;115:105197. doi: 10.1016/j.bioorg.2021.105197. Epub 2021 Jul 21. Bioorg Chem. 2021. PMID: 34426159
-
Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR.Eur J Med Chem. 2021 Dec 5;225:113804. doi: 10.1016/j.ejmech.2021.113804. Epub 2021 Aug 27. Eur J Med Chem. 2021. PMID: 34479036 Review.
-
5-Lipoxygenase as a drug target: A review on trends in inhibitors structural design, SAR and mechanism based approach.Bioorg Med Chem. 2019 Sep 1;27(17):3745-3759. doi: 10.1016/j.bmc.2019.06.040. Epub 2019 Jul 4. Bioorg Med Chem. 2019. PMID: 31331653 Review.
Cited by
-
Effects of some anti-ulcer and anti-inflammatory natural products on cyclooxygenase and lipoxygenase enzymes: insights from in silico analysis.In Silico Pharmacol. 2024 Nov 2;12(2):97. doi: 10.1007/s40203-024-00269-2. eCollection 2024. In Silico Pharmacol. 2024. PMID: 39498163
-
Sources, morphology, phytochemistry, pharmacology of Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma: a review of the literature.Front Pharmacol. 2023 Aug 31;14:1229963. doi: 10.3389/fphar.2023.1229963. eCollection 2023. Front Pharmacol. 2023. PMID: 37719857 Free PMC article. Review.
-
In Vitro Regulation of Amyloid production in Alzheimer's Disease via Curcumin-Loaded Silver Nanoparticles with Antioxidant and Acetylcholinesterase-Inhibiting Properties.Mol Neurobiol. 2025 Jun 3. doi: 10.1007/s12035-025-05125-8. Online ahead of print. Mol Neurobiol. 2025. PMID: 40459716
-
Integrating Network Pharmacology and In Silico Analysis to Explore the Bioactive Compounds Against Gastric Cancer Treatment.Cureus. 2024 Dec 16;16(12):e75779. doi: 10.7759/cureus.75779. eCollection 2024 Dec. Cureus. 2024. PMID: 39816318 Free PMC article.
-
In Silico and In Vitro Approach for Evaluation of the Anti-Inflammatory and Antioxidant Potential of Mygalin.Int J Mol Sci. 2023 Nov 30;24(23):17019. doi: 10.3390/ijms242317019. Int J Mol Sci. 2023. PMID: 38069341 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

