The ER protein Creld regulates ER-mitochondria contact dynamics and respiratory complex 1 activity
- PMID: 35867795
- PMCID: PMC9307246
- DOI: 10.1126/sciadv.abo0155
The ER protein Creld regulates ER-mitochondria contact dynamics and respiratory complex 1 activity
Abstract
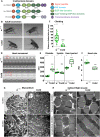
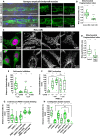
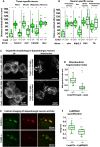
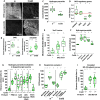
Dynamic contacts are formed between endoplasmic reticulum (ER) and mitochondria that enable the exchange of calcium and phospholipids. Disturbed contacts between ER and mitochondria impair mitochondrial dynamics and are a molecular hallmark of Parkinson's disease, which is also characterized by impaired complex I activity and dopaminergic neuron degeneration. Here, we analyzed the role of cysteine-rich with EGF-like domain (Creld), a poorly characterized risk gene for Parkinson's disease, in the regulation of mitochondrial dynamics and function. We found that loss of Creld leads to mitochondrial hyperfusion and reduced ROS signaling in Drosophila melanogaster, Xenopus tropicalis, and human cells. Creld fly mutants show differences in ER-mitochondria contacts and reduced respiratory complex I activity. The resulting low-hydrogen peroxide levels are linked to disturbed neuronal activity and lead to impaired locomotion, but not neurodegeneration, in Creld mutants. We conclude that Creld regulates ER-mitochondria communication and thereby hydrogen peroxide formation, which is required for normal neuron function.
Figures

References
-
- Ohashi M., Hirano T., Watanabe K., Shoji H., Ohashi N., Baba H., Endo N., Kohno T., Hydrogen peroxide modulates neuronal excitability and membrane properties in ventral horn neurons of the rat spinal cord. Neuroscience 331, 206–220 (2016). - PubMed
-
- Morán M., Moreno-Lastres D., Marín-Buera L., Arenas J., Martín M. A., Ugalde C., Mitochondrial respiratory chain dysfunction: Implications in neurodegeneration. Free Radic. Biol. Med. 53, 595–609 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

