A mechanism of self-lipid endocytosis mediated by the receptor Mincle
- PMID: 35867828
- PMCID: PMC9335232
- DOI: 10.1073/pnas.2120489119
A mechanism of self-lipid endocytosis mediated by the receptor Mincle
Abstract
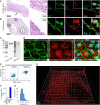
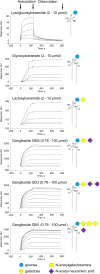
Cellular lipid uptake (through endocytosis) is a basic physiological process. Dysregulation of this process underlies the pathogenesis of diseases such as atherosclerosis, obesity, diabetes, and cancer. However, to date, only some mechanisms of lipid endocytosis have been discovered. Here, we show a previously unknown mechanism of lipid cargo uptake into cells mediated by the receptor Mincle. We found that the receptor Mincle, previously shown to be a pattern recognition receptor of the innate immune system, tightly binds a range of self-lipids. Moreover, we revealed the minimal molecular motif in lipids that is sufficient for Mincle recognition. Superresolution microscopy showed that Mincle forms vesicles in cytoplasm and colocalizes with added fluorescent lipids in endothelial cells but does not colocalize with either clathrin or caveolin-1, and the added lipids were predominantly incorporated in vesicles that expressed Mincle. Using a model of ganglioside GM3 uptake in brain vessel endothelial cells, we show that the knockout of Mincle led to a dramatic decrease in lipid endocytosis. Taken together, our results have revealed a fundamental lipid endocytosis pathway, which we call Mincle-mediated endocytosis (MiME), and indicate a prospective target for the treatment of disorders of lipid metabolism, which are rapidly increasing in prevalence.
Keywords: C-type lectin receptors; Mincle; endocytosis; endothelial cells; ganglioside.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Johannes L., Wunder C., Shafaq-Zadah M., . Glycolipids and Lectins in Endocytic Uptake Processes. Journal of Molecular Biology 428, 4792–4818 (2016). - PubMed
-
- Ferreira A. P. A., Boucrot E., Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 28, 188–200 (2018). - PubMed
-
- Kaksonen M., Roux A., Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018). - PubMed
-
- Kumano-Kuramochi M., et al. , Lectin-like oxidized LDL receptor-1 is palmitoylated and internalizes ligands via caveolae/raft-dependent endocytosis. Biochem. Biophys. Res. Commun. 434, 594–599 (2013). - PubMed
-
- Conway E. M., Boffa M. C., Nowakowski B., Steiner-Mosonyi M., An ultrastructural study of thrombomodulin endocytosis: Internalization occurs via clathrin-coated and non-coated pits. J. Cell. Physiol. 151, 604–612 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

