Divergent evolution of extreme production of variant plant monounsaturated fatty acids
- PMID: 35867834
- PMCID: PMC9335243
- DOI: 10.1073/pnas.2201160119
Divergent evolution of extreme production of variant plant monounsaturated fatty acids
Abstract
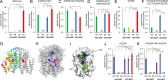
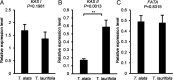
Metabolic extremes provide opportunities to understand enzymatic and metabolic plasticity and biotechnological tools for novel biomaterial production. We discovered that seed oils of many Thunbergia species contain up to 92% of the unusual monounsaturated petroselinic acid (18:1Δ6), one of the highest reported levels for a single fatty acid in plants. Supporting the biosynthetic origin of petroselinic acid, we identified a Δ6-stearoyl-acyl carrier protein (18:0-ACP) desaturase from Thunbergia laurifolia, closely related to a previously identified Δ6-palmitoyl-ACP desaturase that produces sapienic acid (16:1Δ6)-rich oils in Thunbergia alata seeds. Guided by a T. laurifolia desaturase crystal structure obtained in this study, enzyme mutagenesis identified key amino acids for functional divergence of Δ6 desaturases from the archetypal Δ9-18:0-ACP desaturase and mutations that result in nonnative enzyme regiospecificity. Furthermore, we demonstrate the utility of the T. laurifolia desaturase for the production of unusual monounsaturated fatty acids in engineered plant and bacterial hosts. Through stepwise metabolic engineering, we provide evidence that divergent evolution of extreme petroselinic acid and sapienic acid production arises from biosynthetic and metabolic functional specialization and enhanced expression of specific enzymes to accommodate metabolism of atypical substrates.
Keywords: biotechnology; chemical diversity; desaturase; fatty acid; vegetable oil.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Badami R. C., Patil K. B., Structure and occurrence of unusual fatty acids in minor seed oils. Prog. Lipid Res. 19, 119–153 (1980). - PubMed
-
- Ohlrogge J., et al. , PlantFAdb: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 96, 1299–1308 (2018). - PubMed
-
- Smith C. Jr., Occurrence of unusual fatty acids in plants. Prog. Chem. Fats Other Lipids 11, 137–177 (1971).
-
- Cahoon E. B., Li-Beisson Y., Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 55, 66–73 (2020). - PubMed
-
- Stymne S., Ohlrogge J., Tweaking enzymes for exotic plant oils. Nat. Plants 4, 633–634 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

