Shh activation restores interneurons and cognitive function in newborns with intraventricular haemorrhage
- PMID: 35867870
- PMCID: PMC10169407
- DOI: 10.1093/brain/awac271
Shh activation restores interneurons and cognitive function in newborns with intraventricular haemorrhage
Abstract
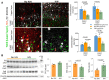
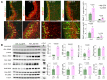
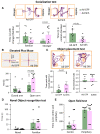
Premature infants with germinal matrix haemorrhage-intraventricular haemorrhage (GMH-IVH) suffer from neurobehavioural deficits as they enter childhood and adolescence. Yet the underlying mechanisms remain unclear. Impaired development and function of interneurons contribute to neuropsychiatric disorders. Therefore, we hypothesized that the occurrence of IVH would reduce interneuron neurogenesis in the medial ganglionic eminence and diminish the population of parvalbumin+ and somatostatin+ cortical interneurons. Because Sonic Hedgehog promotes the production of cortical interneurons, we also postulated that the activation of Sonic Hedgehog signalling might restore neurogenesis, cortical interneuron population, and neurobehavioural function in premature newborns with IVH. These hypotheses were tested in a preterm rabbit model of IVH and autopsy samples from human preterm infants. We compared premature newborns with and without IVH for intraneuronal progenitors, cortical interneurons, transcription factors regulating neurogenesis, single-cell transcriptome of medial ganglionic eminence and neurobehavioural functions. We treated premature rabbit kits with adenovirus expressing Sonic Hedgehog (Ad-Shh) or green fluorescence protein gene to determine the effect of Sonic Hedgehog activation on the interneuron production, cortical interneuron population and neurobehaviour. We discovered that IVH reduced the number of Nkx2.1+ and Dlx2+ progenitors in the medial ganglionic eminence of both humans and rabbits by attenuating their proliferation and inducing apoptosis. Moreover, IVH decreased the population of parvalbumin+ and somatostatin+ neurons in the frontal cortex of both preterm infants and kits relative to controls. Sonic Hedgehog expression and the downstream transcription factors, including Nkx2.1, Mash1, Lhx6 and Sox6, were also reduced in kits with IVH. Consistent with these findings, single-cell transcriptomic analyses of medial ganglionic eminence identified a distinct subpopulation of cells exhibiting perturbation in genes regulating neurogenesis, ciliogenesis, mitochondrial function and MAPK signalling in rabbits with IVH. More importantly, restoration of Sonic Hedgehog level by Ad-Shh treatment ameliorated neurogenesis, cortical interneuron population and neurobehavioural function in kits with IVH. Additionally, Sonic Hedgehog activation alleviated IVH-induced inflammation and several transcriptomic changes in the medial ganglionic eminence. Taken together, IVH reduced intraneuronal production and cortical interneuron population by downregulating Sonic Hedgehog signalling in both preterm rabbits and humans. Notably, activation of Sonic Hedgehog signalling restored interneuron neurogenesis, cortical interneurons and cognitive function in rabbit kits with IVH. These findings highlight disruption in cortical interneurons in IVH and identify a novel therapeutic strategy to restore cortical interneurons and cognitive function in infants with IVH. These studies can accelerate the development of new therapies to enhance the neurodevelopmental outcome of survivors with IVH.
Keywords: Sonic Hedgehog; cortical interneuron; germinal matrix haemorrhage; neurogenesis; premature newborns.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures





Similar articles
-
Duration of culture and sonic hedgehog signaling differentially specify PV versus SST cortical interneuron fates from embryonic stem cells.Development. 2015 Apr 1;142(7):1267-78. doi: 10.1242/dev.111526. Development. 2015. PMID: 25804737 Free PMC article.
-
Disruption of Interneuron Neurogenesis in Premature Newborns and Reversal with Estrogen Treatment.J Neurosci. 2018 Jan 31;38(5):1100-1113. doi: 10.1523/JNEUROSCI.1875-17.2017. Epub 2017 Dec 15. J Neurosci. 2018. PMID: 29246927 Free PMC article.
-
Estrogen Treatment Reverses Prematurity-Induced Disruption in Cortical Interneuron Population.J Neurosci. 2018 Aug 22;38(34):7378-7391. doi: 10.1523/JNEUROSCI.0478-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037831 Free PMC article.
-
Transcriptional Regulation of Cortical Interneuron Development.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 47. PMID: 39637134 Free Books & Documents. Review.
-
The development of MGE-derived cortical interneurons: An Lhx6 tale.Int J Dev Biol. 2022;66(1-2-3):43-49. doi: 10.1387/ijdb.210185md. Int J Dev Biol. 2022. PMID: 34881792 Review.
Cited by
-
Glycogen Synthase Kinase-3β Inhibitor VP3.15 Ameliorates Neurogenesis, Neuronal Loss and Cognitive Impairment in a Model of Germinal Matrix-intraventricular Hemorrhage of the Preterm Newborn.Transl Stroke Res. 2025 Apr;16(2):467-483. doi: 10.1007/s12975-023-01229-2. Epub 2024 Jan 17. Transl Stroke Res. 2025. PMID: 38231413 Free PMC article.
-
New insights and potential biomarkers for intraventricular hemorrhage in extremely premature infant, case-control study.Pediatr Res. 2024 Jul;96(2):395-401. doi: 10.1038/s41390-024-03111-9. Epub 2024 Mar 11. Pediatr Res. 2024. PMID: 38467704
References
-
- Indredavik MS, Skranes JS, Vik T, et al. . Low-birth-weight adolescents: Psychiatric symptoms and cerebral MRI abnormalities. Pediatr Neurol. 2005;33:259–266. - PubMed
-
- Whitaker AH, Feldman JF, Lorenz JM, et al. . Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 2011;68:742–752. - PubMed
-
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. - PubMed

