Modification of the Association Between Frequent Aspirin Use and Ovarian Cancer Risk: A Meta-Analysis Using Individual-Level Data From Two Ovarian Cancer Consortia
- PMID: 35867953
- PMCID: PMC9916035
- DOI: 10.1200/JCO.21.01900
Modification of the Association Between Frequent Aspirin Use and Ovarian Cancer Risk: A Meta-Analysis Using Individual-Level Data From Two Ovarian Cancer Consortia
Abstract
Purpose: Frequent aspirin use has been associated with reduced ovarian cancer risk, but no study has comprehensively assessed for effect modification. We leveraged harmonized, individual-level data from 17 studies to examine the association between frequent aspirin use and ovarian cancer risk, overall and across subgroups of women with other ovarian cancer risk factors.
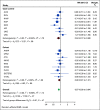
Methods: Nine cohort studies from the Ovarian Cancer Cohort Consortium (n = 2,600 cases) and eight case-control studies from the Ovarian Cancer Association Consortium (n = 5,726 cases) were included. We used Cox regression and logistic regression to assess study-specific associations between frequent aspirin use (≥ 6 days/week) and ovarian cancer risk and combined study-specific estimates using random-effects meta-analysis. We conducted analyses within subgroups defined by individual ovarian cancer risk factors (endometriosis, obesity, family history of breast/ovarian cancer, nulliparity, oral contraceptive use, and tubal ligation) and by number of risk factors (0, 1, and ≥ 2).
Results: Overall, frequent aspirin use was associated with a 13% reduction in ovarian cancer risk (95% CI, 6 to 20), with no significant heterogeneity by study design (P = .48) or histotype (P = .60). Although no association was observed among women with endometriosis, consistent risk reductions were observed among all other subgroups defined by ovarian cancer risk factors (relative risks ranging from 0.79 to 0.93, all P-heterogeneity > .05), including women with ≥ 2 risk factors (relative risk, 0.81; 95% CI, 0.73 to 0.90).
Conclusion: This study, the largest to-date on aspirin use and ovarian cancer, provides evidence that frequent aspirin use is associated with lower ovarian cancer risk regardless of the presence of most other ovarian cancer risk factors. Risk reductions were also observed among women with multiple risk factors, providing proof of principle that chemoprevention programs with frequent aspirin use could target higher-risk subgroups.
Conflict of interest statement
Modification of the Association Between Frequent Aspirin Use and Ovarian Cancer Risk: A Meta-Analysis Using Individual-Level Data From Two Ovarian Cancer Consortia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- American Cancer Society . Cancer Facts & Figures 2021. Atlanta, GA: 2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
-
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459–1467. - PubMed
-
- Fathalla MF. Incessant ovulation—A factor in ovarian neoplasia? Lancet. 1971;2:163. - PubMed
-
- Moorman PG, Schildkraut JM, Calingaert B, et al. Ovulation and ovarian cancer: A comparison of two methods for calculating lifetime ovulatory cycles (United States) Cancer Causes Control. 2002;13:807–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN261201800032C/CA/NCI NIH HHS/United States
- HHSN261201800009C/CA/NCI NIH HHS/United States
- N01 PC067010/PC/NCI NIH HHS/United States
- K05 CA154337/CA/NCI NIH HHS/United States
- R01 CA058860/CA/NCI NIH HHS/United States
- HHSN261201800015I/CA/NCI NIH HHS/United States
- K07 CA092044/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- R01 CA049449/CA/NCI NIH HHS/United States
- HHSN261201800015C/CA/NCI NIH HHS/United States
- CRUK_/Cancer Research UK/United Kingdom
- HHSN261201800009I/CA/NCI NIH HHS/United States
- U01 CA199277/CA/NCI NIH HHS/United States
- UM1 CA164917/CA/NCI NIH HHS/United States
- NU58DP006344/DP/NCCDPHP CDC HHS/United States
- R01 CA087538/CA/NCI NIH HHS/United States
- Z01 ES044005/ImNIH/Intramural NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- R01 CA067262/CA/NCI NIH HHS/United States
- R01 CA095023/CA/NCI NIH HHS/United States
- R03 CA113148/CA/NCI NIH HHS/United States
- R01 CA058598/CA/NCI NIH HHS/United States
- U01 CA176726/CA/NCI NIH HHS/United States
- P30 CA014089/CA/NCI NIH HHS/United States
- R01 CA039742/CA/NCI NIH HHS/United States
- R03 CA115195/CA/NCI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- R01 AG061132/AG/NIA NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- R01 CA076016/CA/NCI NIH HHS/United States
- R01 CA077398/CA/NCI NIH HHS/United States
- HHSN261201800032I/CA/NCI NIH HHS/United States
- P01 CA017054/CA/NCI NIH HHS/United States
- N01 CN025403/CA/NCI NIH HHS/United States
- P30 CA071789/CA/NCI NIH HHS/United States
- R01 CA112523/CA/NCI NIH HHS/United States

