3D genome, on repeat: Higher-order folding principles of the heterochromatinized repetitive genome
- PMID: 35868274
- PMCID: PMC10225251
- DOI: 10.1016/j.cell.2022.06.052
3D genome, on repeat: Higher-order folding principles of the heterochromatinized repetitive genome
Abstract
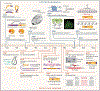
Nearly half of the human genome is comprised of diverse repetitive sequences ranging from satellite repeats to retrotransposable elements. Such sequences are susceptible to stepwise expansions, duplications, inversions, and recombination events which can compromise genome function. In this review, we discuss the higher-order folding mechanisms of compartmentalization and loop extrusion and how they shape, and are shaped by, heterochromatin. Using primarily mammalian model systems, we contrast mechanisms governing H3K9me3-mediated heterochromatinization of the repetitive genome and highlight emerging links between repetitive elements and chromatin folding.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
Long interspersed nuclear element 1 and B1/Alu repeats blueprint genome compartmentalization.Curr Opin Genet Dev. 2023 Jun;80:102049. doi: 10.1016/j.gde.2023.102049. Epub 2023 May 23. Curr Opin Genet Dev. 2023. PMID: 37229928 Review.
-
Heterochromatin: Guardian of the Genome.Annu Rev Cell Dev Biol. 2018 Oct 6;34:265-288. doi: 10.1146/annurev-cellbio-100617-062653. Epub 2018 Jul 25. Annu Rev Cell Dev Biol. 2018. PMID: 30044650 Review.
-
Special Issue: Repetitive DNA Sequences.Genes (Basel). 2019 Nov 6;10(11):896. doi: 10.3390/genes10110896. Genes (Basel). 2019. PMID: 31698818 Free PMC article.
-
The molecular basis of the organization of repetitive DNA-containing constitutive heterochromatin in mammals.Chromosome Res. 2017 Mar;25(1):77-87. doi: 10.1007/s10577-016-9547-3. Epub 2017 Jan 11. Chromosome Res. 2017. PMID: 28078514 Review.
-
Candida albicans repetitive elements display epigenetic diversity and plasticity.Sci Rep. 2016 Mar 14;6:22989. doi: 10.1038/srep22989. Sci Rep. 2016. PMID: 26971880 Free PMC article.
Cited by
-
PML is a constitutive component of chromatin domains enriched in repetitive elements and duplicated gene clusters in cancer cells.Heliyon. 2024 Aug 17;10(17):e36499. doi: 10.1016/j.heliyon.2024.e36499. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39263139 Free PMC article.
-
ZMYM2 controls human transposable element transcription through distinct co-regulatory complexes.Elife. 2023 Nov 7;12:RP86669. doi: 10.7554/eLife.86669. Elife. 2023. PMID: 37934570 Free PMC article.
-
Polygenic burden of short tandem repeat expansions promotes risk for Alzheimer's disease.Nat Commun. 2025 Jan 28;16(1):1126. doi: 10.1038/s41467-025-56400-0. Nat Commun. 2025. PMID: 39875385 Free PMC article.
-
Intrinsic catalytic properties of histone H3 lysine-9 methyltransferases preserve monomethylation levels under low S-adenosylmethionine.J Biol Chem. 2023 Jul;299(7):104938. doi: 10.1016/j.jbc.2023.104938. Epub 2023 Jun 17. J Biol Chem. 2023. PMID: 37331600 Free PMC article.
-
Transposable elements in mammalian chromatin organization.Nat Rev Genet. 2023 Oct;24(10):712-723. doi: 10.1038/s41576-023-00609-6. Epub 2023 Jun 7. Nat Rev Genet. 2023. PMID: 37286742 Review.
References
-
- Achour M, Jacq X, Rondé P, Alhosin M, Charlot C, Chataigneau T, Jeanblanc M, Macaluso M, Giordano A, Hughes AD, et al. (2008). The interaction of the SRA domain of ICBP90 with a novel domain of DNMT1 is involved in the regulation of VEGF gene expression. Oncogene 27, 2187–2197. 10.1038/sj.onc.1210855. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

