T-bet+ B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity
- PMID: 35868310
- PMCID: PMC9357106
- DOI: 10.1016/j.cmet.2022.07.002
T-bet+ B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity
Abstract
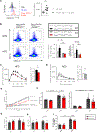
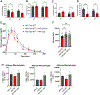
Obesity is accompanied by inflammation in adipose tissue, impaired glucose tolerance, and changes in adipose leukocyte populations. These studies of adipose tissue from humans and mice revealed that increased frequencies of T-bet+ B cells in adipose tissue depend on invariant NKT cells and correlate with weight gain during obesity. Transfer of B cells enriched for T-bet+ cells exacerbates metabolic disorder in obesity, while ablation of Tbx21 specifically in B cells reduces serum IgG2c levels, inflammatory cytokines, and inflammatory macrophages in adipose tissue, ameliorating metabolic symptoms. Furthermore, transfer of serum or purified IgG from HFD mice restores metabolic disease in T-bet+ B cell-deficient mice, confirming T-bet+ B cell-derived IgG as a key mediator of inflammation during obesity. Together, these findings reveal an important pathological role for T-bet+ B cells that should inform future immunotherapy design in type 2 diabetes and other inflammatory conditions.
Keywords: B cells; CD11c(+) T-bet(+) B cells; IgG2c; adipose tissue; glucose intolerance; iNKT cells; inflammation; metabolic disorder; obesity; type 2 diabetes.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing financial interests.
Figures





Comment in
-
T-bet B or not T-bet B, is that the question in obesity-related metabolic disease?Cell Metab. 2022 Aug 2;34(8):1081-1082. doi: 10.1016/j.cmet.2022.07.008. Cell Metab. 2022. PMID: 35921814
-
T-bet+ B cells promote metabolic disease.Nat Rev Endocrinol. 2022 Oct;18(10):589. doi: 10.1038/s41574-022-00736-w. Nat Rev Endocrinol. 2022. PMID: 35970892 No abstract available.
-
T-bet+ B cells exacerbate obesity-related metabolic disease.Trends Immunol. 2022 Nov;43(11):855-857. doi: 10.1016/j.it.2022.09.011. Epub 2022 Oct 7. Trends Immunol. 2022. PMID: 36216716
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

